Structure of the Atom NCERT Questions Solution
1. Compare the properties of electrons, protons and neutrons.
Answer: Comparison between properties of electrons, protons and neutrons-
| Charge | Mass (amu) | Location | |
|---|---|---|---|
| Proton | +1e | 1 | nucleus |
| Neutron | 0 | 1 | nucleus |
| Electron | -1e | negligible | orbitals |
2. What are the limitations of J.J. Thomson’s model of the atom?
Limitation: It could not explain the origin of spectral series of H atom and also result of Rutherford α -Particle Scattering Experiment.
3.What are the limitations of Rutherford’s model of the atom?
Limitations of Rutherford’s Atomic Model:
(i) Rutherford’s atomic model cannot explain the stability of an atom.
(ii) In Rutherford’s atomic model an electron can revolve in orbits of all possible radii .so it should emit a continuous spectrum. But an atom like hydrogen always emits a discrete line spectrum.
4. Describe Bohr’s model of the atom.
Bohr’s Atomic Model
(i) An atom consists of a small and massive central core, called Nucleus; electrons revolve around nucleus in circular orbit like planetary motion.
(ii) Only certain special orbits known as discrete orbits (non-radiating orbits or Stationary Orbits) of electrons, are allowed inside the atom. While revolving in discrete orbits the electrons do not radiate energy.
(iii) An atom can emit or absorb radiation only when an electron jumps from one orbit to another orbit.
Table of Contents

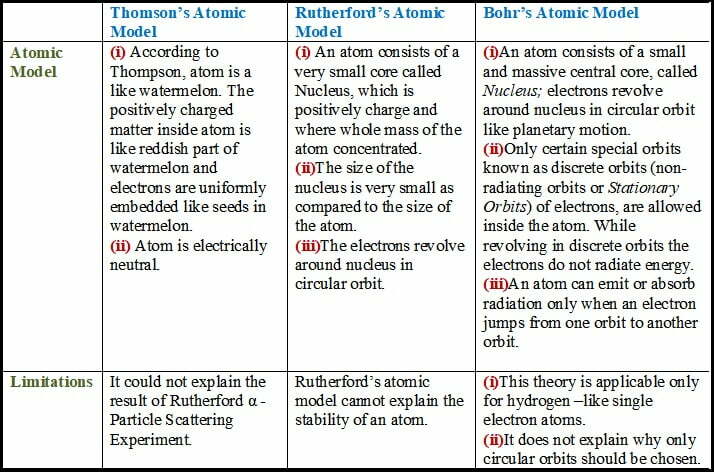
5. Compare all the proposed models of an atom given in this chapter.
6.Summarise the rules for writing of distribution of electrons in various shells for the first eighteen elements.
According to Bohr and Bury, following rules are used for distribution of electrons in different energy levels or shells for the first eighteen elements:
(i) Maximum number of electrons that can be present in a shell is determined by the formula 2n2, where ‘n’ – orbit number or energy level index, 1,2,3,….
So, maximum number of electrons in different shells will be,
First orbit(n=1) or K-shell will be = 2 × 12 = 2,
Second orbit(n=2) or L-shell will be = 2 × 22 = 8,
Third orbit(n=3) or M-shell will be = 2 × 32 = 18,
Fourth orbit(n=4) or N-shell will be = 2 × 42= 32, and so on.
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Filling of electrons in shells is done in step-wise manner i.e. Electrons are not accommodated in a given shell, unless the inner shells are empty (not completely filled). 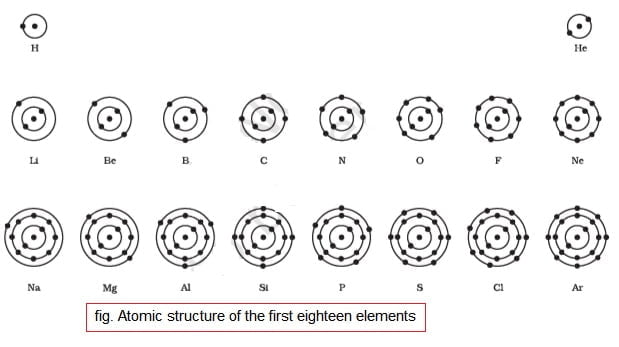
7. Define valency by taking examples of silicon and oxygen.
VALENCY : “Valency of an atom is definite combining capacity of an atom with other atoms when it forms chemical compounds/ molecules or number of that electrons which atom loses/accept to complete it’s octoate.”
Valency of an atom is determined by
(i) If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell.
Distribution of electrons in Si (Z = 14) = 2,8.4
Since, outermost shell have 4 electrons so, The valency of Silicon = 4
(ii) If the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
Distribution of electrons in O (Z = 8) = 2,6
Since, outermost shell have 6 electrons so, The valency of Oxygen = 8 – 6 = 2
8. Explain with examples (i) Atomic number (ii) Mass number (iii) Isotopes and (iv) Isobars. Give any two uses of Isotopes.
(i) Atomic number (Z): The total number of protons in an atom is equal to atomic number, which is also equal to number of electrons in that atom at neutral state.
For example, the atomic number of Helium is 2. It means He contains 2 protons and 2 electrons.
(ii) Mass number (A): Mass number of an atom is equal to total number of protons and neutrons present in a nucleus.
Mass number of an atom = Number of protons + Number of neutrons
For example, the atomic number of Sodium is 11 which, is equal to the number of protons, the number of neutrons in Sodium is 12.
So, The mass number of Sodium is equal to 23 (11+12).
(iii) Isotopes: The atoms of an element which have the same atomic number but different mass number are called isotopes.
For example, Hydrogen has three isotopes:
Hydrogen Protium (1H1) – its nucleus has one proton;
Deuterium (1H2)-its nucleus has one proton and one neutron;
and Tritium (1H3) –its nucleus has one proton and two neutrons.
Uses of Isotopes:
(a) Isotopes of Uranium are used in Nuclear reactor.
(b) Isotopes of Carbon are used in Radio Carbon dating.
(c) An isotope of Cobalt is used in the treatment of cancer.
(iv) Isobars: The atoms having the same mass number (A) but different atomic number.
For example: (17Cl37) and (16S37), as both have same mass number = 37.
(20Ca40) and(18Ar40), as both have same A = 40.
9. Na+ has completely filled K and L shells. Explain.
Atomic number of Sodium Z=11, sodium ion Na+, has 10 electrons in it Na ——–→ Na+ + 1e-(released electron).
Electronic configuration of Na+is Na+= 2,8
The maximum capacity of K shell is 2 electrons and that of L shell is 8 electrons. Since, total number of electrons required to fill both shell completely is 2 + 8 = 10 electrons and Na+ also have 10 electrons . So, sodium ion Na+ has completely filled K and L shells.
10.If bromine atom is available in the form of, say, two isotopes 35Br78(49.7%) and 35Br81(50.3%), calculate the average atomic mass of bromine atom.
ANS: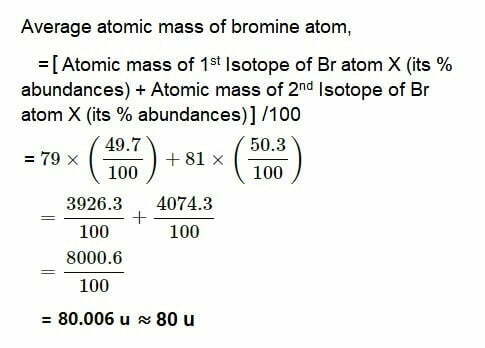
11. The average atomic mass of a sample of an element X is 16.2 u. What are the percentages of isotopes 8X16 and 8X18in the sample?
ANS: 
12. If Z = 3, what would be the valency of the element? Also, name the element.
ANS: Name of element is Lithium (Li) as it’s atomic number is Z=3.
Distribution of electrons in Li (Z = 3) = 2,1
(Concept: If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell.)
Since, outermost shell have 1 electrons so,The valency of Lithium = 1
13. Composition of the nuclei of two atomic species X and Y are given as under, – X Y Protons = 6 6 Neutrons = 6 8 Give the mass numbers of X and Y. What is the relation between the two species?
ANS: Mass number of an atom = Number of protons + Number of neutrons
Mass number of X = 6 + 6 = 12 Carbon
Mass number of Y = 6 + 8 = 14 Carbon
Since, atomic number (same protons) of X and Y is same but their mass numbers are different so both are Isotopes.
14. For the following statements, write T for True and F for False.
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
(c) The mass of an electron is about 1/2000 times that of proton.
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine
(a) J.J. Thomson proposed that the nucleus of an atom contains only nucleons.(F)
(b) A neutron is formed by an electron and a proton combining together. Therefore, it is neutral. (F)
(c) The mass of an electron is about 1/2000 times that of proton. (T)
(d) An isotope of iodine is used for making tincture iodine, which is used as a medicine. (T)
☞ Put tick (☑) against correct choice and cross (☒) against wrong choice in questions 15, 16 and 17.
15. Rutherford’s alpha-particle scattering experiment was responsible for the discovery of
(a) Atomic Nucleus
(b) Electron
(c) Proton
(d) Neutron
ANS:(a) Atomic Nucleus (☑)
(b) Electron(☒)
(c) Proton(☒)
(d) Neutron (☒)
16. Isotopes of an element have
(a) the same physical properties
(b) different chemical properties
(c) different number of neutrons
(d) different atomic numbers.
ANS: (a) the same physical properties (☒)
(b) different chemical properties (☒)
(c) different number of neutrons(☑)
(d) different atomic numbers. (☒)
17. Number of valence electrons in (Cl–) ion are:
(a) 16
(b) 8
(c) 17
(d) 18
ANS: (a) 16 ☒
(b) 8 (☑)
(c) 17 ☒
(d) 18 ☒
18.Which one of the following is a correct electronic configuration of sodium?
(a) 2,8
(b) 8,2,1
(c) 2,1,8
(d) 2,8,1.
ANS: (a) 2,8 (☒)
(b) 8,2,1 (☒)
(c) 2,1,8 (☒)
(d) 2,8,1 (☑)
19. Complete the following table.
ANS: 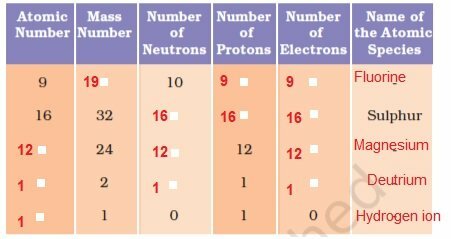
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution
Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution, Structure of the Atom NCERT Questions Solution