Chapter-04 Structure of the Atom NCERT in-text questions
Structure of the Atom NCERT in-text questions page 47
1. What are canal rays?
Answer: Canal rays are beam of positively charge particles. They are also known as Anode rays. It was discovered by E. Goldstein in 1886 in a gas discharge tube.
2. If an atom contains one electron and one proton, will it carry any charge or not?
Answer: There will be no charge (neutral) on atom as charge on electron and proton is equal and opposite.
Table of Contents
Structure of the Atom NCERT in-text questions page 49
1.On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
Answer: According to J. J. Thompson, an atom consists of a positively charged sphere and the negatively charged electrons are embedded in it. The negative and positive charges are equal in magnitude. So, the atom as a whole is electrically neutral.
2. On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
Answer: On the basis of Rutherford’s model of an atom proton is subatomic particle which is present inside nucleus of an atom.
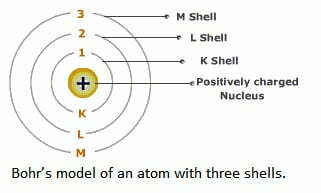
3. Draw a sketch of Bohr’s model of an atom with three shells.
4.What do you think would be the observation, if the a-particle scattering experiment is carried out using a foil of a metal other than gold?
Answer: If Rutherford’s α-particle scattering experiment is carried out using a foil of other metal rather than gold then we will also get similar observations. Since, other metals foil can’t make thin as gold so more α-particles will rebound and we do not get good result as using thin foil of gold(highly malleable).
1.Name the three sub-atomic particles of an atom.
Answer: Three sub-atomic particles of an atom are (i) Electron (ii) Proton (iii) Neutron
2. Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Answer: Atomic mass = Number of protons+ Number of neutrons
4 = 2 + Number of neutrons
Number of neutrons = 2
Structure of the Atom NCERT in-text questions page 50
1.Write the distribution of electrons in carbon and sodium atoms.
Answer: For Carbon atom-
Carbon atomic number = 6
It’s electronic configuration is C = 1s2, 2s2, 2p2
So, electrons will be distributed as,
In the inner most orbit (n=1, 1s2 ) or K-shell = 2 electrons
In the second orbit (n=2, 2s2 2p2) or L-shell = 4 electrons For Sodium atom-
Sodium atomic number = 11
It’s electronic configuration is Na = 1s2 2s2 2p6 3s1
So, electrons will be distributed as,
In the inner most orbit (n=1, 1s2 ) or K-shell = 2 electrons
In the second orbit (n=2, 2s2 2p6 ) or L-shell = 8 electrons
In the third orbit (n=3, 3s1 ) or M-shell = 1 electron
2. If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Answer: Maximum number of electrons in K-shell = 2 electrons
Maximum number of electrons in L-shell = 8 electrons
Total number of electrons in the atom = 2 + 8 = 10 electrons
Structure of the Atom NCERT in-text questions page 52
1. How will you find the valency of Chlorine, Sulphur and Magnesium?
Answer: “Valency of an atom is definite combining capacity of an atom with other atoms when it forms chemical compounds/ molecules or number of that electrons which atom loses/accept to complete it’s octoate.”
Valency of an atom is determined by
(i) If the number of electrons in the outermost shell of the atom of an element is less than or equal to 4, then the valency of the element is equal to the number of electrons in the outermost shell.
Distribution of electrons in Mg (Z = 10) = 2,8.2
Since, outermost shell have 2 electrons so, The valency of Magnesium (Mg) = 2
(ii) If the number of electrons in the outermost shell of the atom of an element is greater than 4, then the valency of that element is determined by subtracting the number of electrons in the outermost shell from 8.
Distribution of electrons in Cl (Z = 17) = 2,8.7
Since, outermost shell have 7 electrons so, The valency of Chlorine (Cl) = 8 −7 = 1
Distribution of electrons in S (Z = 16) = 2,8.6
Since, outermost shell have 6 electrons so, The valency of Sulphur (S) = 8 − 6 = 2
1.If number of electrons in an atom is 8 and number of protons is also 8, then (i) what is the atomic number of the atom? and (ii) what is the charge on the atom?
Answer: (i) Atomic number = number of electrons = number of protons = 8
(ii) Charge on the atom = 0 (If number of electrons is equal to number of protons in an atom then total charge on atom is zero )
2. With the help of Table 4.1, find out the mass number of Oxygen and Sulphur atom.
Answer: Mass number of Oxygen atom = 16
Mass number of Sulphur atom = 32
Structure of the Atom NCERT in-text questions page 53
1. For the symbol H,D and T tabulate three sub-atomic particles found in each of them.
Answer: Three main sub-atomic particles are protons, electrons and neutrons.
| Hydrogen Isotopes | Number of Electrons | Number of Protons | Number of Neutrons | Mass Number |
|---|---|---|---|---|
| Protium (H) | 1 | 1 | 0 | 1 |
| Deuterium (D) | 1 | 1 | 1 | 2 |
| Tritium (T) | 1 | 1 | 1 | 3 |
2. Write the electronic configuration of any one pair of isotopes and isobars.
Answer: Electronic configuration of a pair of isotopes (for isotope of CARBON), same electronic configuration
6C12 = 1s2, 2s2, 2p2
6C14 = 1s2, 2s2, 2p2
Electronic configuration of a pair of isobars (Ca and Ar),
20Ca40 = 2, 8, 8, 2
18Ar40 = 2, 8, 8
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Structure of the Atom NCERT in-text questions , Structure of the Atom NCERT in-text questions page 53