Hello, readers ! Here you learn about the properties of the photon. The photon picture of EM radiation. ‘Dual Nature of Matter’. Like radiation, matter also shows dual behavior in particle as well as wave nature.
Properties of Photon based on Particle theory of Light
- The photon picture of electromagnetic radiation is as follows:
- When radiation interacts with matter, it behaves as if it is made up of particles called photons.
- A photon’s rest mass is zero, while its dynamic or kinetic mass is
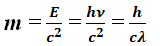
- Each photon has energy and momentum, and moves at the speed of light c.
- All photons of light of the same frequency (ν) or same wavelength (λ), have the same energy (E = hν = hc/ λ) and momentum (p= hν/c = h/λ), whatever the intensity of radiation may be. By increasing the intensity of light at a given wavelength, there is only an increase in the number of photons per second crossing a given area, with each photon having the same energy. Thus, photon energy is independent of the intensity of radiation.
- Photons are electrically neutral and are not deflected by electric and magnetic fields.
- In a photon-particle collision (such as a photon-electron collision), the total energy and total momentum are conserved. However, the number of photons may not be conserved in a collision. The photon may be absorbed or a new photon may be created.
Table of Contents
Dual Nature of Matter
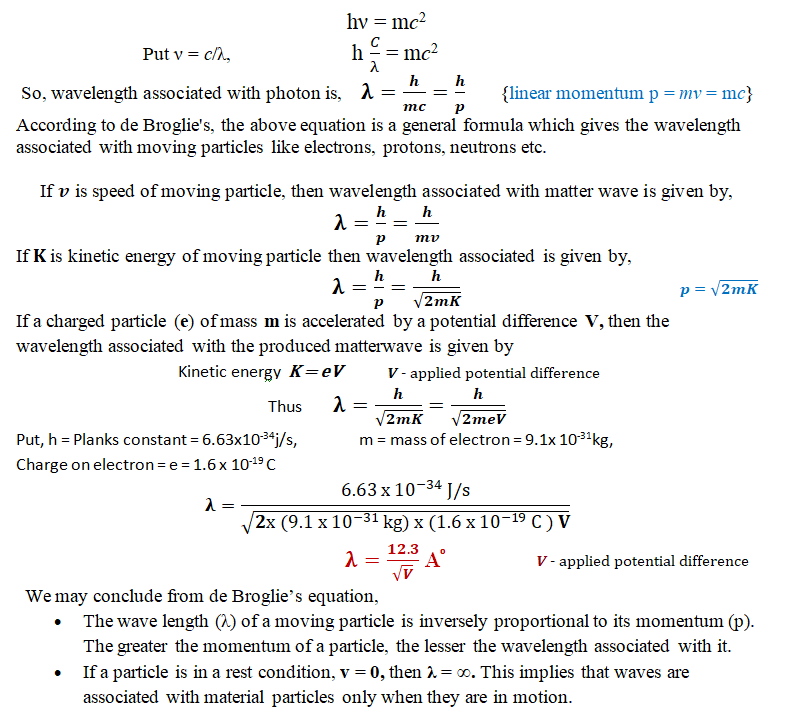
In 1924, Louis Victor de Broglie hypothesized that material particles in motion should display wave-like properties as well as matter. He reasoned that nature was symmetrical and that the two fundamental physical entities—matter and energy—must also be symmetrical. If radiation has two sides, wave as well as particle (photon), then so should matter. The waves associated with material particles in motion are called matter waves or de Broglie waves, and their wave length is called the de-Broglie wave length.
The de-Broglie wavelength is given by λ = h/p, where λ is the particle’s wavelength, h is Planck’s constant, and p is the particle’s momentum.
de-Broglie’s equation
The de Broglie’s equation is a fundamental principle of quantum mechanics that relates the wavelength of a particle to its momentum. The equation is given by λ = h/p, where λ is the particle’s wavelength, h is Planck’s constant, and p is the particle’s momentum.
Considering beam of photons as a wave, the energy of a photon from Planck’s quantum theory is given by
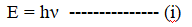
Considering a photon as a particle of mass m, its energy is given by Einstein’s mass-energy equivalence.
E= mc2 ————— (ii)
Comparing equation (i) and (ii),
Important questions from the topic
What is a photon?
Ans: A photon is a fundamental particle of light and other forms of electromagnetic radiation. It is a type of boson, which means it does not obey the Pauli exclusion principle and can exist in the same quantum state as other photons.
What are the properties of a photon?
Ans: Some of the properties of a photon include its energy, wavelength, frequency, and momentum. A photon has no mass and travels at the speed of light in a vacuum. Its energy is directly proportional to its frequency and inversely proportional to its wavelength.
How does a photon interact with matter?
Ans: A photon can interact with matter through a variety of processes, including absorption, reflection, scattering, and transmission. When a photon is absorbed, its energy is transferred to the absorbing material, which may result in the emission of a new photon. When a photon is reflected, it bounces off the surface of the material. When a photon is scattered, it may change direction and/or lose some of its energy. When a photon is transmitted, it passes through the material without being absorbed, reflected, or scattered.
How are photons produced?
Ans: Photons can be produced in a variety of ways, including through the acceleration of charged particles, such as electrons, through atomic transitions, and through the annihilation of particle-antiparticle pairs. In addition, photons can be produced through thermal radiation, which is the emission of electromagnetic radiation by a material due to its temperature.
What are some applications of photons in modern technology?
Ans: Photons have many practical applications in modern technology. For example, they are used in fiber optic communication systems to transmit information over long distances. They are also used in solar panels to convert sunlight into electrical energy, in medical imaging technologies such as X-rays and MRI, and in electronic devices such as cameras, barcode scanners, and printers. In addition, photons are used in many scientific experiments to study the properties of matter and the behavior of particles at the atomic and subatomic level.
Can photons be destroyed or created?
Ans: Photons are a type of elementary particle, which means they cannot be created or destroyed in isolation. However, they can be absorbed or emitted by matter, which can change their frequency and energy. In some cases, photons can also be converted into other forms of energy, such as heat or electricity.
Can photons have different shapes or sizes?
Ans: Photons are elementary particles and do not have a shape or size in the traditional sense. However, they do have properties such as energy, frequency, and wavelength, which can vary depending on their source and interactions with matter.
Can photons be entangled?
Ans: Yes, photons can be entangled, which means that the quantum state of one photon is correlated with the quantum state of another photon, even if they are separated by a large distance. This property of entanglement has been used to develop new technologies such as quantum cryptography and quantum computing.
How does the wave-particle duality apply to photons?
Ans: The wave-particle duality is a fundamental concept in quantum mechanics that describes how particles can exhibit both wave-like and particle-like behavior depending on the experiment being performed. This concept applies to photons as well, as they can exhibit wave-like behavior, such as interference patterns, or particle-like behavior, such as being absorbed or emitted by matter.
Are photons affected by gravity?
Ans: Yes, photons are affected by gravity and are subject to the same laws of general relativity as other particles. This means that they can be deflected or redshifted by strong gravitational fields, such as those near massive objects like black holes. This effect has been observed in astronomical observations and is an important prediction of general relativity.
What is the Dual Nature of Matter?
Ans: The Dual Nature of Matter refers to the idea that all matter exhibits both wave-like and particle-like behavior. This concept is a fundamental principle of quantum mechanics and was first proposed by Louis de Broglie in 1924.
How is the Dual Nature of Matter demonstrated in experiments?
Ans: The Dual Nature of Matter is demonstrated in experiments such as the double-slit experiment, which shows that particles such as electrons can exhibit interference patterns similar to those of waves. Other experiments, such as diffraction and scattering experiments, also demonstrate the wave-like behavior of particles.
What is the significance of the Dual Nature of Matter?
Ans: The Dual Nature of Matter is significant because it challenges traditional ideas of the nature of matter and helps to explain phenomena that cannot be explained by classical mechanics. It also led to the development of quantum mechanics, which has had a profound impact on modern science and technology.
How is the Dual Nature of Matter related to the Uncertainty Principle?
Ans: The Dual Nature of Matter is related to the Uncertainty Principle, which states that the more precisely the position of a particle is known, the less precisely its momentum can be known, and vice versa. This principle arises from the wave-particle duality of matter, which means that particles have both wave-like and particle-like properties.
What are some examples of everyday objects that exhibit the Dual Nature of Matter?
Ans: Everyday objects that exhibit the Dual Nature of Matter include electrons, which are fundamental particles that exhibit both wave-like and particle-like behavior. Other examples include photons, which are particles of light, and atoms and molecules, which can exhibit wave-like behavior in certain circumstances.
What are some practical applications of the Dual Nature of Matter?
Ans: The Dual Nature of Matter has many practical applications in modern science and technology, including in the development of semiconductors, quantum computing, and medical imaging technologies such as MRI. It also underlies many of the principles of modern physics, including the Standard Model of particle physics and the theory of relativity.
What is de Broglie equation?
Ans: The de Broglie’s equation is a fundamental principle of quantum mechanics that relates the wavelength of a particle to its momentum. The equation is given by λ = h/p, where λ is the particle’s wavelength, h is Planck’s constant, and p is the particle’s momentum.
What does de Broglie’s equation tell us about the behavior of particles?
Ans: The de Broglie equation tells us that particles, including electrons and other subatomic particles, have both wave-like and particle-like properties. This duality is a fundamental concept in quantum mechanics and can be observed in experiments such as the double-slit experiment.
What is the significance of de Broglie’s equation?
Ans: De Broglie’s equation is significant because it shows that matter can exhibit wave-like behavior, which was previously thought to be exclusive to electromagnetic radiation. This discovery helped to establish the principles of quantum mechanics and led to the development of new technologies such as the electron microscope and the semiconductor transistor.
How is de Broglie’s equation related to Heisenberg’s uncertainty principle?
Ans: De Broglie’s equation is related to Heisenberg’s uncertainty principle, which states that the more precisely the position of a particle is known, the less precisely its momentum can be known, and vice versa. De Broglie’s equation shows that the wavelength of a particle is inversely proportional to its momentum, which means that the more precisely the momentum of a particle is known, the less precisely its position can be known, and vice versa.
Can de Broglie’s equation be applied to macroscopic objects?
Ans: The de Broglie’s equation can be applied to macroscopic objects in theory, but the wavelengths involved are extremely small and difficult to observe. The wavelength of a macroscopic object, such as a baseball, would be so small that it would be practically impossible to measure in practice. Therefore, de Broglie’s equation is generally considered to be applicable only to subatomic particles.
What does de Broglie’s equation tell us about the behavior of particles?
Ans: The de Broglie equation tells us that particles, including electrons and other subatomic particles, have both wave-like and particle-like properties. This duality is a fundamental concept in quantum mechanics and can be observed in experiments such as the double-slit experiment.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon, Properties of Photon