Photoelectric Effect
The phenomenon in which electrons are emitted from a metallic surface, when high energy electromagnetic radiation falls on it is called photoelectric effect.
The phenomenon of photoelectric emission was first discovered in 1887 by Heinrich Hertz (1857-1894) and later on by Wilhelm Hallwachs and Philipp Lenard, who investigated the phenomenon of photoelectric emission in detail during 1886-1902.
Hertz’s Experiment
The phenomenon of photoelectric effect was first discovered by Heinrich hertz in 1887 while performing an experiment for the production of EM-wave. He observed that when U-V light falls on the smooth metal surface (detector) electrons are emitted, which produces sparks across the detector loop.
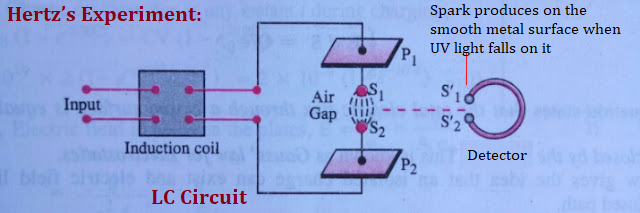
When high energy radiation U-V falls on a metal surface, some electrons near the surface absorb energy from the incident radiation to overcome the binding energy of electrons. After gaining sufficient energy from the incident light, the electrons escape from the surface of the metal into the surrounding space. This effect was later called the photoelectric effect.
Table of Contents
Hallwach’s and Lenard’s observations
Wilhelm Hallwach’s and Philipp Lenard study the photoelectric effect during1886-1902.The experimental arrangement consists of an evacuated glass/quartz tube which encloses a photosensitive (Zn) plate C(emitter) and another metal plate A(collector). The two plates are connected to high tension battery. The battery maintains the potential difference between the plates C and A, that can be varied. The polarity of the plates C and A can be reversed by a device called commutator. When monochromatic radiations of suitable frequency falls on the plate C, electrons are emitted which are collected by plate A, so the current produced called photoelectric current. The potential difference between the emitter and collector plates is measured by a voltmeter (V) whereas the resulting photoelectric current flowing in the circuit is measured by a microammeter (μA).
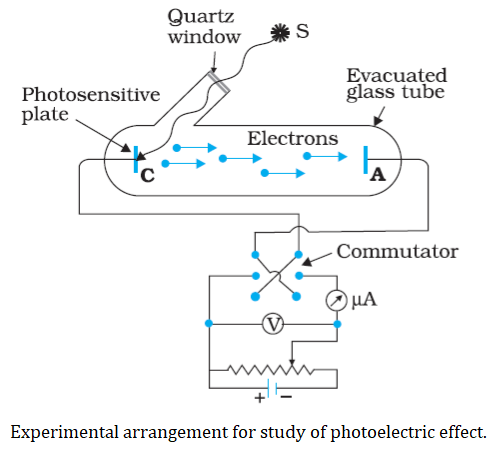
- Some terms you should know before further study
- Photoelectric current: the current produced by the emission of photoelectrons as a result of the photoelectric effect. The greater the number of photoelectrons emitted per unit time, the greater the photoelectric current.
- Frequency of radiation: The frequency of radiation is related to the energy of each photon. The greater the frequency of radiation, the more energetic photons will be.
- Threshold frequency: The minimum value of frequency of incident radiation which is just capable of ejecting an electron from metal surface is called Threshold/Cut–off Frequency, below this frequency photoelectric emission stops.
- Intensity of radiation: The amount of energy falling on a surface per unit time per unit area (Unit-W/m2) is referred to as radiation intensity. The intensity of radiation increases with an increase in the number of photons falling per unit time.
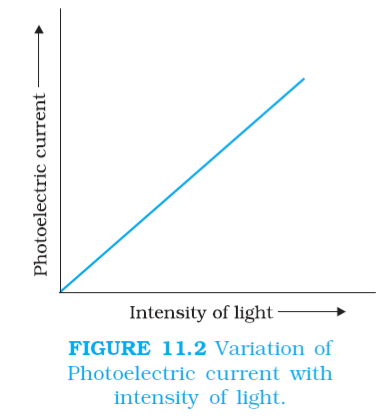
(1) Effect of intensity of light on photoelectric current:
When collector (A) is maintained at a positive potential and emitter C at a negative potential, then at a constant suitable frequency above the threshold frequency, photoelectric current is directly proportional to the intensity of incident radiation.
(2) Effect of Potential on photoelectric current:
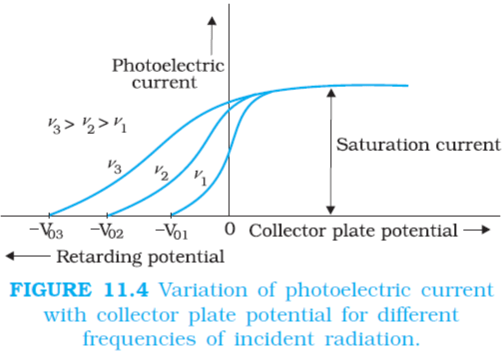
When Plate A is maintained positive and plate C is negative, for fixed intensity and for constant frequency. The photoelectric current increases with the increases in accelerating potential till a stage is reached when the photoelectric current become maximum and does not increases further with increases in the accelerating potential. This maximum value of current is called Saturation Current.
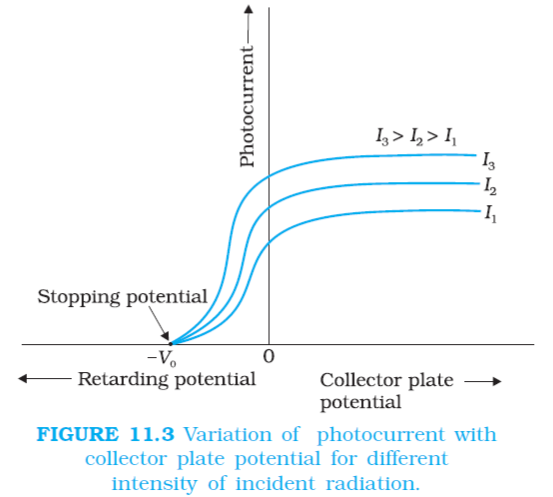
If we apply negative potential on plate A with respect to plate C, and increase its magnitude gradually then the photoelectric current decreases rapidly and become zero. The value of negative/retarding potential at which the photoelectric current becomes zero is called Cut-Off or Stopping Potential (Vₒ). Photoelectric current becomes zero because the stopping potential is sufficient to attract even the most energetic photoelectrons to emit, with the maximum kinetic energy (Kmax), so that
Kmax = e Vₒ
If we increase the intensity of incident radiation at same frequency (above threshold value) the values of saturation current also increases due to increase in number of photoelectrons emitted while, stopping potential and maximum kinetic energy of photoelectrons is independent of this intensity.
(3) Effect of frequency of incident radiation on stopping potential:
At constant intensity of radiation the value of stopping potential increases with increase in frequency of incident radiation, while there is no change in saturation current.
Graph between the frequency of incident radiation and corresponding stopping potential:
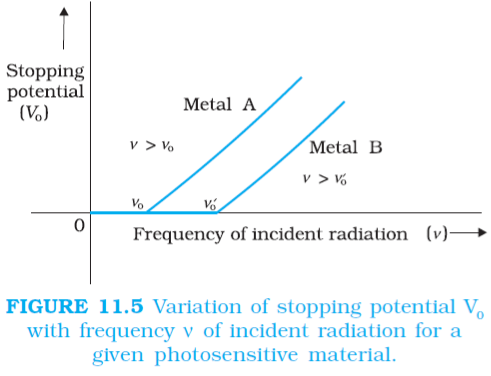
This graph reveals following observation and conclusion,
| Sr. No. | Observation | Conclusion |
|---|---|---|
| 1 | For a given photosensitive material, stopping potential increases linearly with incident radiation frequency | The maximum kinetic energy of the photoelectrons varies linearly with the frequency of incident radiation but is independent of its intensity. |
| 2 | A minimum cut-off frequency exists for which the stopping potential is zero | For a frequency ν of incident radiation lower than the cut-off frequency |
| 3 | For two different metals, A and B, the threshold frequency is different. |
Law of Photoelectric Emission:
On the basis of above experiment it is observed that,
- For a given photosensitive material and frequency of incident radiation (above the threshold frequency), the photoelectric current is directly proportional to the intensity of light and saturation current is directly proportional to the intensity of incident radiation.
- For a given photosensitive material, there exists a threshold frequency below which no photoelectrons are emitted; however high is the intensity of incident radiation.
- Above the threshold frequency, the stopping potential or equivalently the maximum kinetic energy of the photoelectrons is directly proportional to the frequency of incident radiation, but is independent of its intensity.
- The photoelectric effect is instantaneous process.
Important Questions from Photoelectric Effect
What is the photoelectric effect?
Answer: The photoelectric effect is the phenomenon in which electrons are emitted from a material when it is exposed to electromagnetic radiation such as light.
Who discovered the photoelectric effect?
Answer: The photoelectric effect was first observed and studied by Heinrich Hertz in the late 19th century, and later explained by Albert Einstein in 1905.
What is the threshold frequency in the photoelectric effect?
Answer: The threshold frequency is the minimum frequency of light required to eject electrons from a metal surface in the photoelectric effect.
What is the stopping potential in the photoelectric effect?
Answer: The stopping potential is the minimum potential difference required to stop the flow of emitted electrons in the photoelectric effect.
How does the energy of incident photons affect the photoelectric effect?
Answer: The energy of incident photons must exceed the work function of the material in order to cause electron emission in the photoelectric effect. Higher energy photons can eject electrons with greater kinetic energy.
How does the intensity of light affect the photoelectric effect?
Answer: The intensity of light affects the number of electrons emitted, but not their energy. This is because the energy of each photon is determined solely by its frequency, not its intensity.
What were Hallwachs’ observations?
Answer: In 1888, Philipp Lenard and Wilhelm Hallwachs observed that a negatively charged electrode, known as the collector, attracted electrons emitted by a photoelectrically charged zinc plate. They found that the kinetic energy of the emitted electrons was proportional to the frequency of the incident light, and that there was a minimum frequency below which no electrons were emitted.
Q: What did Hallwachs conclude from his observations? A: Hallwachs concluded that electrons are emitted from a material when it is exposed to light and that the energy of the emitted electrons is related to the frequency of the incident light.
Q: What were Lenard’s observations? A: In 1902, Philipp Lenard observed that the maximum kinetic energy of the emitted electrons was not affected by the intensity of the incident light, but only by its frequency. He also found that the stopping potential required to stop the flow
Q: Who was Hallwachs and what were his observations? A: Philipp Lenard was a German physicist who conducted experiments in the late 19th and early 20th century. He observed that when ultraviolet (UV) light was shone on a metal surface, electrons were emitted from the surface. This phenomenon is known as the photoelectric effect.
Q: What did Hallwachs observe in relation to the photoelectric effect? A: Hallwachs observed that the photoelectric effect is dependent on the material from which the metal surface is made. Different materials emit electrons with different energies when exposed to UV light.
Q: What is Lenard known for in relation to the photoelectric effect? A: Lenard is known for his work in characterizing the photoelectric effect. He discovered that the kinetic energy of the emitted electrons was dependent on the frequency of the incident UV light, not its intensity.
Q: What did Lenard’s observations reveal about the nature of light? A: Lenard’s observations suggested that light behaves as both a wave and a particle. The frequency of the light wave determines the energy of the individual particles (photons) that make up the light. This concept would eventually lead to the development of quantum mechanics.
Q: How did Hallwachs and Lenard’s observations contribute to the understanding of the photoelectric effect? A: Hallwachs and Lenard’s observations helped to establish the relationship between the frequency of light and the kinetic energy of emitted electrons. This relationship could not be explained by classical physics and played a key role in the development of quantum mechanics.
Q: How did Hallwachs’ observation contribute to the understanding of the photoelectric effect? A: Hallwachs’ observation that the photoelectric effect is dependent on the material from which the metal surface is made helped to establish that electrons are tightly bound to atoms within a material, and that the energy required to free them varies from material to material.
Q: How did Lenard’s observation contribute to the understanding of the photoelectric effect? A: Lenard’s observation that the kinetic energy of the emitted electrons is dependent on the frequency of the incident light, and not its intensity, helped to establish the particle-like nature of light and led to the development of quantum mechanics.
What are the applications of photoelectric effect?
Answer: The photoelectric effect is used in a variety of technologies, such as solar cells, photodiodes, and imaging devices.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ