Photoelectric Effect and Wave Theory of Light
The wave theory of light is unable to explain the most basic features of photoelectric effects like one-to-one electron-photon interaction and no time lag in photoelectric emission for the following reasons.
According to the wave theory of light,
- Free electrons at the surface of the metal (over which the beam of radiation falls) absorb the radiant energy continuously. The greater the intensity of radiation, the greater the energy absorbed by each electron. No matter what the frequency of radiation is. A threshold frequency, therefore, should not exist. These expectations of the wave theory directly contradict observations (i),(ii) and (iii) as observed by Wilhelm Hallwach and Philipp Lenard during their study of the photoelectric effect.
- The energy transfer by radiation to electrons, it does not go to a particular electron but it is distributed uniformly to all electrons. Therefore, it takes time to collect the energy required for the ejection of an electron, but experimental observation shows that there is no time lag.
Thus, the wave theory of light is unable to explain the most basic features of photoelectric emission.
Table of Contents

Dual Nature of Radiation:
The dual nature of radiation here basically means the ‘wave nature’ and the ‘particle nature’. Some natural phenomena like interference, diffraction, and polarisation that occur in nature can be explained only when we consider light as a wave. Other phenomena, such as the photoelectric effect, can only be explained when light is viewed as a particle. Such particles of light are called photons.
Radiation has dual nature and can be explained on the basis of two theories,
- Wave theory of radiation: According to this theory radiation is an electromagnetic wave, which travels with speed 3×108m/s. Some phenomenon like interference, diffraction and polarisation can be explained on the basis of wave theory.
- Quantum theory of radiation/light: According to this theory radiation is composed of particle which moves in straight line with very high speed. Phenomenon like reflection, refraction, photoelectric effect, Compton Effect can be explained on the basis of this.
Important questions from Dual Nature of Radiation and Matter
Explain of Photoelectric Effect on the basis of Einstein’s photoelectric equation.
Einstein’s photoelectric equation, 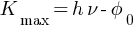
Einstein’s photoelectric equation explains the photoelectric effect with various parameters,
- Explanation of effect of intensity: With increase in intensity of light (no. of photon increases) number of photoelectrons increases which increases photoelectric current.
- Explanation of the threshold frequency:
- If ν < νₒ i.e. the frequency of incident radiation (ν) is less than the threshold frequency (νₒ). The photoelectric emission does not occur.
- If ν > νₒ is the frequency of incident radiation is more than the threshold frequency then the maximum kinetic energy of the electrons increases.
- Explanation of time lag: There is no time lag between the incidence of photon and the emission of a photoelectron (less than 10-9s).
Other Important questions
Question 3: Why is the wave theory of light not able to explain the observed features of the photoelectric effect? Explain
Question 4: Every metal has a definite work function, why do photoelectrons not come out with the same energy of incident radiation if incident radiation is monochromatic? Why is there an energy distribution of photo electrons?
Q: What is the photoelectric effect? A: The photoelectric effect is the phenomenon where electrons are emitted from the surface of a material when it is exposed to light.
Q: How does the photoelectric effect challenge the wave theory of light? A: The photoelectric effect challenged the wave theory of light because the classical wave theory predicted that increasing the intensity of light would increase the number of ejected electrons, while the energy of each electron would remain constant. However, the experimental data showed that the energy of the ejected electrons varied with the frequency of light, and not its intensity.
Q: What did Einstein propose in his explanation of the photoelectric effect? A: Einstein proposed that light consists of discrete packets of energy called photons, and that the energy of each photon is directly proportional to its frequency. He used this concept to explain the dependence of the kinetic energy of the ejected electrons on the frequency of light, and not its intensity.
Q: How did Einstein’s proposal reconcile the wave theory of light with the photoelectric effect? A: Einstein’s proposal of the particle-like nature of light reconciled the wave theory of light with the photoelectric effect by explaining how photons with different frequencies (corresponding to different colors) could have different energies, and could therefore eject electrons from a material with different energies.
Q: What is the wave theory of light? A: The wave theory of light is the concept that light travels as a wave, with oscillating electric and magnetic fields. It was developed by Huygens, Fresnel, and Maxwell in the 17th, 18th, and 19th centuries.
Q: What are some phenomena that can be explained by the wave theory of light? A: The wave theory of light can explain phenomena such as interference, diffraction, and polarization, as well as the color and brightness of light. It also provides a good description of the behavior of light over long distances and on large scales.
Q: How is the wave theory of light used in modern physics? A: The wave theory of light is still used in modern physics to describe the behavior of light in many situations, including optics, astronomy, and quantum mechanics. However, the concept of light as a particle (or photon) is also crucial to understanding many phenomena, such as the photoelectric effect and the Compton effect.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light, Photoelectric Effect and Wave Theory of Light