Hello, readers 😊! Here you can learn about p-type semiconductor. A pure semiconductor doped with a trivalent impurity like Al, Ga, In etc. gives an p-type semiconductor.
Table of Contents
p-type semiconductor
p-type semiconductor is obtained when pure Si or Ge crystal is doped with a trivalent impurity like Al, Ga, In, etc.
The dopant atom has 3 valence electrons, one valence electron less than Si or Ge. The dopant atom can form covalent bonds with three neighbouring Si/Ge atoms, but it does not have an extra electron to make a bond with the fourth Si atom. So there is a vacancy between the fourth neighbour Si/Ge and the trivalent dopant atom. This vacancy is known as a hole, which can accept an electron, and this trivalent atom is called an acceptor atom.
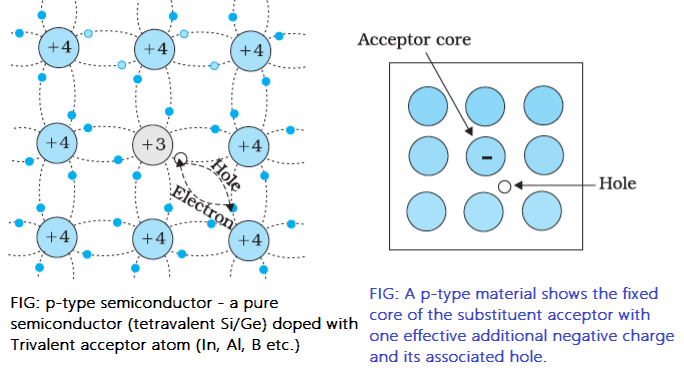
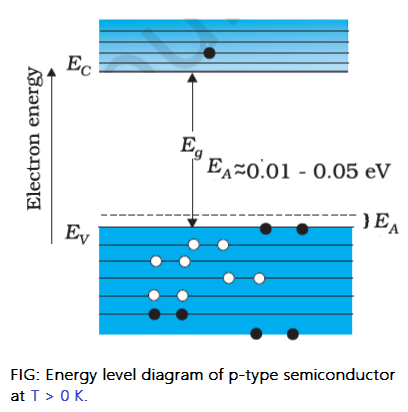
The energy level in which the acceptor atom accepts electrons from the valance band of Si is called the acceptor level (EA) One acceptor atom gives one hole for conduction. These holes are in addition to the intrinsically generated holes, while the source of conduction electrons is only intrinsic generation. Thus, for this type of material, the holes are the majority carriers, and electrons are the minority carriers. Therefore, extrinsic semiconductors doped with trivalent impurities are called p-type semiconductors.
Important questions from Topic
What is a p-type semiconductor?
Ans: A p-type semiconductor is a type of semiconductor material in which the majority charge carriers are holes, which are positively charged vacancies in the valence band of the material. P-type semiconductors are created by doping an intrinsic or lightly doped semiconductor material with impurities that have one less valence electron than the host material.
What are some common impurities used for doping p-type semiconductors?
Ans: Boron, aluminum, and gallium are commonly used impurities for doping p-type semiconductors. These elements have only three valence electrons, and when they are incorporated into a semiconductor lattice, they create holes in the valence band that act as the majority carriers.
How does doping affect the electronic properties of a semiconductor?
Ans: Doping introduces impurity atoms into a semiconductor lattice, which can either donate or accept electrons to change the conductivity and electronic properties of the material. P-type doping creates a material with a net positive charge and an excess of holes in the valence band, making it a positive-type conductor.
What are some practical applications of p-type semiconductors?
Ans: P-type semiconductors are used in a wide variety of electronic devices, including diodes, transistors, and solar cells. They are often used in conjunction with n-type semiconductors to form p-n junctions, which have unique electronic properties that are useful for a range of applications.
How does a p-type semiconductor differ from an n-type semiconductor?
Ans: An n-type semiconductor is doped with impurities that have one more valence electron than the host material, creating excess electrons in the conduction band that act as the majority carriers. In contrast, a p-type semiconductor is doped with impurities that have one less valence electron than the host material, creating excess holes in the valence band that act as the majority carriers. When a p-type and n-type semiconductor are brought into contact, they form a p-n junction with unique electronic properties.
How is the conductivity of a p-type semiconductor affected by temperature?
Ans: Like all semiconductors, the conductivity of a p-type semiconductor increases with temperature due to increased thermal energy that promotes the formation of additional charge carriers. However, the temperature dependence of the conductivity is more complex than for metals or insulators, as it depends on both the intrinsic properties of the material and the effects of doping.
What is an n-type semiconductor?
Ans: An n-type semiconductor is a type of semiconductor material in which the majority charge carriers are electrons, which are negatively charged particles that occupy the conduction band of the material. N-type semiconductors are created by doping an intrinsic or lightly doped semiconductor material with impurities that have one more valence electron than the host material.
What are some common impurities used for doping n-type semiconductors?
Ans: Phosphorus, arsenic, and antimony are commonly used impurities for doping n-type semiconductors. These elements have five valence electrons, and when they are incorporated into a semiconductor lattice, they create excess electrons in the conduction band that act as the majority carriers.