Hello, readers! Here you learn about how energy bands are formed in solids? What are the energy levels? What are Energy bands? An energy band is a collection of closely spaced energy levels. How energy bands are formed in solids?
How energy bands are formed in solids?
In an isolated atom (separate from each other by a large distance), each electron has discrete energies and ‘quantized’ in different orbits, but the electron energy is the same in each orbit ‘Energy levels‘.
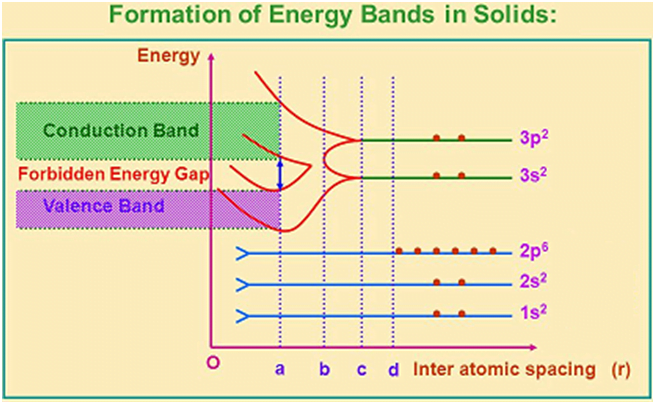
However, because the atoms in a crystal are close to each other (2 to 3 Å), the electrons of one atom interact with the electrons and nucleus of the other atom. The interaction is more felt by the electrons in the outermost orbit, while the inner orbit electron energies may remain unaffected. Therefore, due to inter-atomic interaction, their energy levels are modified. These modified energy levels with continuous energy variation form energy bands.
Two types of energy band in solids:
- Valance band: The energy band associated with the energy levels of the valence electrons is called the valence band. This band is always filled completely or partially with electrons, but can never be empty. Valence electrons are not capable of gaining energy from an external electric field so that they can participate in the conductivity of electric current.
- Conduction band: The energy band above the valence band is called the conduction band. The conduction band is either empty or partially filled. Conduction band electrons are capable of gaining energy from an external electric field and participate in the conductivity of electric current.
In some solids, there is some energy gap between the conduction band and the valence band, called the Forbidden energy gap (Eg). For example, the energy gap for Si is 1.1 eV, 0.70 eV for Ge, and 5.4 eV for carbon. The forbidden energy gap is completely empty, having no electrons.
In some solids, electrons of the valence band may gain energy from an external source and cross the energy gap to reach the conduction band, where they can now participate in conductivity.
Table of Contents
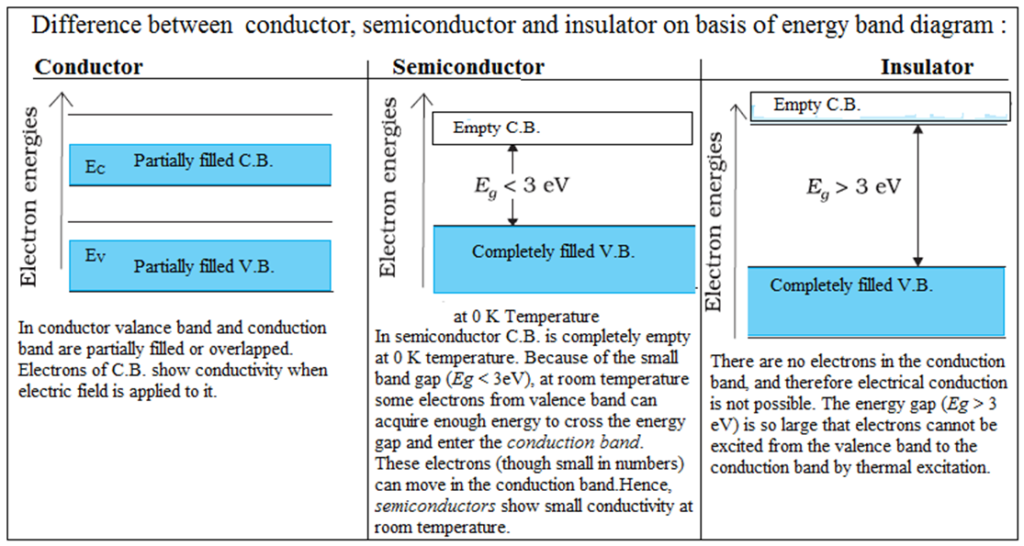
Difference between conductors, semiconductors and insulators on the basis of Energy band diagram
Important questions based on Energy Band
What are the energy levels?
The energies of electrons while revolving in each of the allowed orbitals are called Energy levels.
What is the band gap of a semiconductor?
Answer: The band gap of a semiconductor is the energy difference between its valence band (where electrons are held in place by atoms) and its conduction band (where electrons can move freely and conduct electricity). This gap determines the conductivity and other electrical properties of the material.
What are energy bands in solids? How do they relate to the electronic structure of materials?
Energy bands in solids refer to the range of energies that electrons in a material can occupy. An energy band is a collection of closely spaced energy levels. They are formed from the overlapping of atomic orbitals in a solid, which gives rise to delocalized electronic states that can span a range of energies.
How does the concept of a crystal lattice influence the formation of energy bands in solids?
The concept of a crystal lattice is central to the formation of energy bands in solids, as it provides a periodic array of atoms that can interact with one another to form electronic states. The symmetry of the lattice can influence the size and shape of the energy bands, as well as the electronic and optical properties of the material.
How does the size of the energy band gap relate to the electrical conductivity of a material?
The size of the energy band gap is a key determinant of the electrical conductivity of a material. Materials with smaller band gaps tend to be more conductive, as they allow electrons to move more easily between the valence and conduction bands. Materials with larger band gaps are generally insulating, as they require a larger amount of energy to excite electrons from the valence to the conduction band.
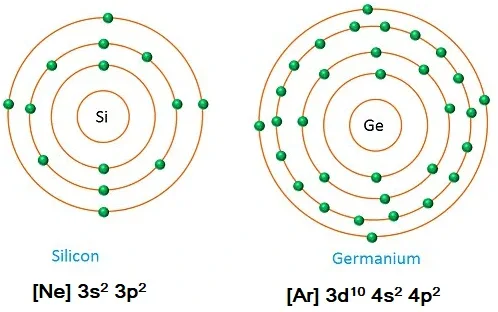
Why is Germanium preferred over Silicon for making semiconductor devices?
Answer:
Germanium is preferred over Silicon because the energy gap for germanium is 0.7 electron volts, while that for silicon is 1.1 electron volts. The laser energy gap between the conduction band and the valence band increases the conductivity of semiconductor material. This translates to a lower “cut-in” voltage for Ge when compared to Si.
More the electrons in the orbits, it is easier to detach electrons from the atom with less energy. Germanium has more electrons, and it is easier to lose electrons at lower voltages.
How energy bands are formed in solids?, How energy bands are formed in solids?, How energy bands are formed in solids?, How energy bands are formed in solids?, How energy bands are formed in solids?, How energy bands are formed in solids?