ELECTRICITY Chapter 12
| Syllabus Electricity: Electric current, potential difference and electric current. Ohm’s law; Resistance, Resistivity, Factors on which the resistance of a conductor depends. Series combination of resistors, parallel combination of resistors and its applications in daily life. Heating effect of electric current and its applications in daily life. Electric power, Interrelation between P, V, I and R. |
CHARGE:
Charge is the property of a material due to which an electrostatic force acts between two charge bodies.
Unit of Charge:
The SI unit of charge is C (coulomb).
1 coulomb (C) of charge is equivalent to the charge contained in nearly 6 × 1018 electrons.
Properties of Charges:
# Charge is quantized, i.e., charge on a body is equal to the integral multiple of the charge on one electron.
Q = ± ne
n = 1, 2, 3, …………..
e = -1.6 ×10-19 C
# Charge on one electron is equal to -1.6 ×10-19 coulomb.
# Charge on electrons and protons have equal magnitudes but opposite sign.
Charge on one Electron = – Charge on one Proton
# An atom is always neutral, as it contains an equal number of electrons and protons. An atom becomes charged / ionised when it loses or gains electrons, and in that case, the number of electrons is not equal to the number of protons. For example:
K (neutral atom) ——–→ K+ (positive Ion) + 1e– (released electron)
Types of charge:
Charge is of two types (1) Positive charge (2) Negative charge
(1) Positive charge: If a body gives/release electrons, it becomes positively charge.
For example: Na (neutral) ——–→ Na+ (positively charge) + 1e–(released electron)
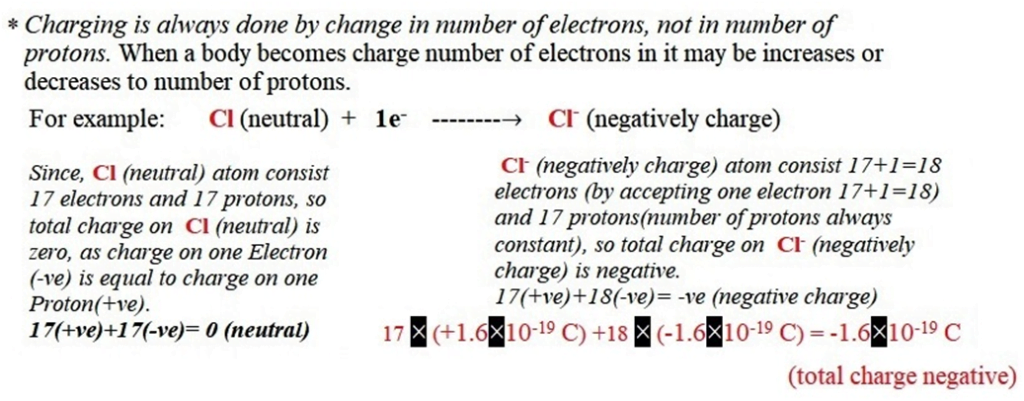
(2) Negative charge: If a body accepts electrons, it becomes negatively charge.
For example: Cl (neutral) +1e–——–→ Cl– (negatively charge)
Key Concept ☞ : The assignment of positive charge is purely a convention. It doesn’t mean that negative charge is less than positive charge i.e. +2C and -2C are same amount of charge. +2C of charge means body acquires this charge by donating/releasing electrons, while,-2C of charge means body acquires this charge by accepting electrons.
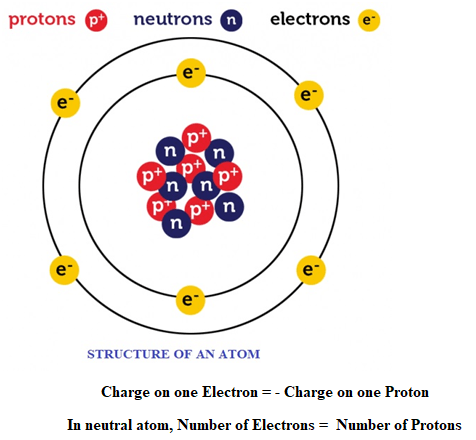
About atom: All matters surrounding us are made from atoms. Before we study more about charge we should know some basic concept of atom. An atom consists of mainly 3 subatomic particles namely Proton, Electron and Neutron.
- Protons: It is positively charge particle, which lies in nucleus of an atom. Charge on one proton is +1.6×10-19 C.
- Electron: It is negatively charge particle, which revolve surrounding nucleus of an atom. Charge on one electron is -1.6×10-19 C.
- Neutron: It is a neutral (charge less) particle which lies inside nucleus.
Table of Contents
Electrostatic force:
Electrostatic force is the force acting between two charged bodies. Electrostatic force is both attractive and repulsive in nature. Unlike charges attract each other, while like charges repel each other.

Electric current:
- The rate of flow of electric charge is called electric current.
- I = Q / t Q = Amount of charge flowing t = time taken
- Current is scalar quantity. It’s SI unit is ampere(A). I = Q / t 1ampere=1coulomb/1second 1A = 1C/s
- Definition of 1 ampere: The amount of current flowing through a wire is said to be 1 ampere if 1coulomb of charge is flowing through it in 1 second.
- Current is measured by an instrument called Ampere meter(=Ammeter). Ammeter is always connected in series in circuit.
A current of 0.5 A is drawn by a filament of an electric bulb for 10 minutes. Find the amount of electric charge that flows through the circuit.
Answer: Given, I = 0.5 A; t = 10 min = 600 s.
From formula, Q = It
Q = 0.5 A × 600 s
Q = 300 C
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
ELECTRICITY Chapter 12, ELECTRICITY Chapter 12, ELECTRICITY Chapter 12, ELECTRICITY Chapter 12, ELECTRICITY Chapter 12