Hello, readers😊! Here we will learn about Einstein Photoelectric Equation. This equation explains the photoelectric effect and the particle nature of light.
Einstein Photoelectric Equation
Einstein’s Photoelectric Equation
In 1905, Albert Einstein (1879-1955) proposed that,
- Photoelectric emission does not take place by continuous absorption of energy from radiation. Radiation energy is built up of discrete units called quanta of energy of radiation. Each quantum of radiant energy has energy hν, where h is Planck’s constant and ν the frequency of light.
- Photoelectrons are emitted as a result of interaction between photon of incident radiation and an electron of photosensitive metal.
- Each photon interacts with one electron. The energy (E= hν) of the incident photon is used up in two parts,
- A part of the energy of the photon is used in liberating the electron from the metal surface, which is equal to the work function ɸₒ of the metal.
- The remaining energy of the photon is used in imparting kinetic energy to the ejected electron.
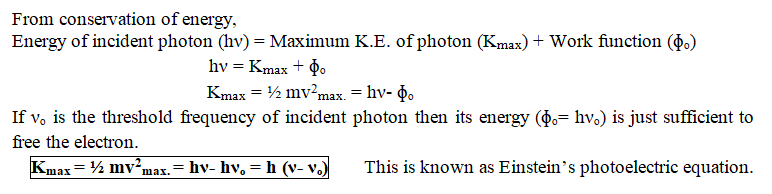
Table of Contents
Explanation of Photoelectric Effect on the basis of Einstein’s photoelectric equation:
- Explanation of effect of intensity: With increase in intensity of light (no. of photon increases) number of photoelectrons increases which increases photoelectric current.
- Explanation of the threshold frequency:
- If ν < νₒ i.e. the frequency of incident radiation (ν) is less than the threshold frequency (νₒ). The photoelectric emission does not occur.
- If ν > νₒ is the frequency of incident radiation is more than the threshold frequency then the maximum kinetic energy of the electrons increases.
- Explanation of time lag: There is no time lag between the incidence of photon and the emission of a photoelectron(less than 10-9s).
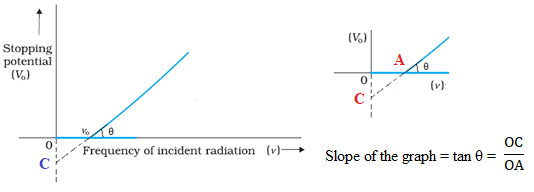
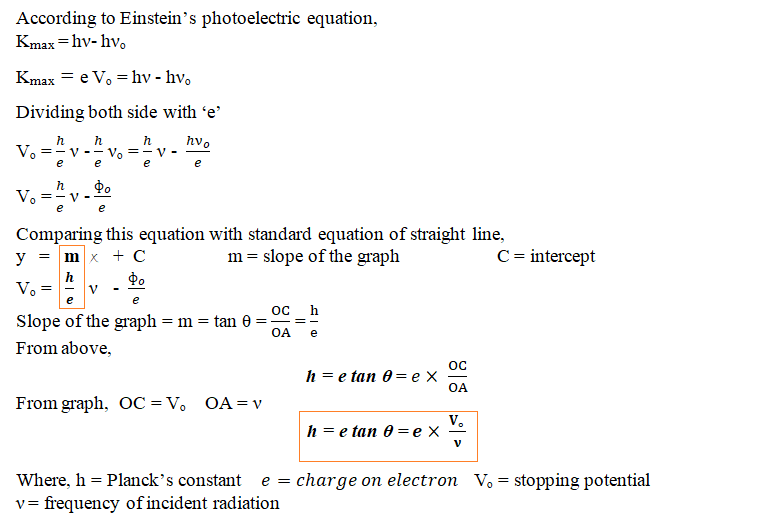
Determine the value of the Planck’s constant and work function from the graph between stopping potential and frequency of incident radiation.
Answer:
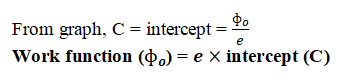
Important questions from topic
What is Einstein’s photoelectric equation?
Answer: Einstein’s photoelectric equation is a formula that describes the relationship between the energy of a photon and the amount of energy needed to remove an electron from a metal surface. The equation states that the energy of a photon (E) is equal to the work function of the metal (W) plus the kinetic energy of the emitted electron (K), or E = W + K.
Q: What does the photoelectric equation explain?
A: The photoelectric equation explains the photoelectric effect, which is the phenomenon in which electrons are emitted from a metal surface when it is exposed to electromagnetic radiation such as light. The equation explains that the energy of a photon must be greater than the work function of the metal in order to eject an electron, and that any excess energy is converted into the kinetic energy of the emitted electron.
Q: How did Einstein’s photoelectric equation help to reconcile the wave-particle duality of light?
A: Einstein’s photoelectric equation helped to reconcile the wave-particle duality of light by showing that electromagnetic radiation, such as light, behaves as both a wave and a particle. The equation showed that photons, which are particles of light, carry discrete packets of energy, which is a characteristic of particles. At the same time, the photoelectric effect shows that the behavior of light can be explained in terms of waves, as the energy of the photons is related to their frequency, which is a characteristic of waves.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation, Einstein Photoelectric Equation