Hello, readers 😊! Here you learn about the distance of closest approach. How did Rutherford estimated the size of the nucleus?
Table of Contents
Distance of Closest Approach (Estimation of size of Nucleus)
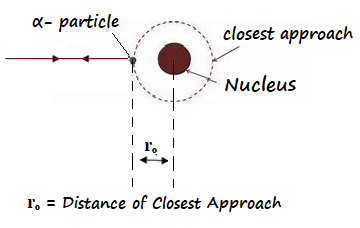
It may be defined as the distance between the centre of the nucleus and the point from which an α- particle approaching directly to the nucleus bounces back.
Expression for Distance of Closest Approach: Consider an α-particle of mass m moving with velocity v and approaching the nucleus. It experiences Coulombic repulsion and its kinetic energy gets converted into electrostatic potential energy. At a certain distance from the nucleus, α- particle stops and rebounces back along its path this distance (rₒ) is called Distance of Closest Approach.
Kinetic energy of α-particle approaching the nucleus, (K) = 1/2 mv2
Electrostatic Potential energy of α-particle at Distance of Closest Approach, (U) = k q1q2 / ro
Charge on of α-particle q1 = 2e, Charge on Gold nucleus q2 = Ze
Putting above values in expression of Potential Energy, U = k q1q2 / ro = k 2e Ze / ro = = 2 k Z e2 / ro
From energy conservation, K = U ⇒ K = 2 k Z e2 / ro
ro = 2 k Z e2 / K
Putting value of K, from experimental data and other parameters also, K = 5.5 MeV = 5.5 × 106 × 1.6 × 10-19J, Gold Atomic number (Z) = 79, k (Coulomb’s constant) = 9 × 109 Nm2/C2
rₒ = 2 k Z e2 / K = 2 × ( 9 × 109 ) × 79 × (1.6 × 10-19)2 / 5.5 × 106 × 1.6 × 10-19
rₒ = 4.13 × 10-14 m = 41.3 fm
We may conclude that the radius of the nucleus is of the order of one Fermi from the calculated value of the distance of closest approach above.
Impact Parameter
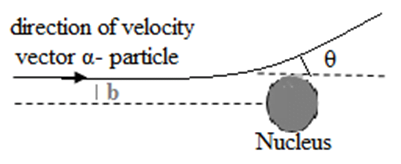
The perpendicular distance of the velocity vector of the α-particle from the line passing through the centre of the nucleus when it is far away from the atom.
The value of the impact parameter determines the shape of the scattered -particle’s trajectory.
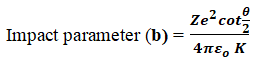
Significance of Impact Parameter:
- If the scattering angle is small, the impact parameter (b) has a large value. i.e., α – particles travelling far away from the nucleus suffer small deflections.
- If the scattering angle is large, the impact parameter (b) has a small value. i.e., α – particles travelling close to the nucleus suffer large deflections.
- If the scattering angle is 180˚, the impact parameter (b) is zero. i.e., The α – particles retrace their path.
Rutherford’s Atomic Model
- An atom consists of a small and massive core called Nucleus, in which the entire positive charge and almost the whole mass of the atom (about 99.99%) are concentrated.
- The size of nucleus (10-15m) is extremely small as comparison to size of atom (10-10m).
- The atom as a whole is electrically neutral because the nucleus is surrounded by an appropriate amount of electrons, whose combined negative charge is equivalent to the nucleus’s combined positive charge.
- The electrons revolve around the nucleus in various orbits, just as planets revolve around the sun. The centripetal force required for their revolution is provided by the electrostatic attraction between the electron and the nucleus.
Limitations of Rutherford’s Atomic Model
- The stability of an atom cannot be explained by Rutherford’s atomic model.
- An electron can rotate in any feasible orbit in Rutherford’s atomic model. Thus, a continuous spectrum should be emitted. However, a discrete line spectrum, not a continuous spectrum, is always emitted by an atom like hydrogen.
Important Questions
What is the order of distance of closest approach in Rutherford’s alpha particle scattering experiment ?
Answer: The order of distance of closest approach in Rutherford’s alpha particle scattering experiment is about few fm (41.3 fm).
An α−particle accelerated through a potential difference of 2 ×106 V and then it strikes on a silver foil. The charge number of silver is 47. Estimate the distance of closest approach of the particle striking to the nucleus.
Answer: Distance of closest approach = 3.76 ×10-15 m = 3.76 fm
When an electron falls from higher energy to a lower energy level, the difference in the energies appear in the form of electromagnetic radiation. Why can’t it be emitted as other forms of energy.
Answer: This is because electron interacts only electromagnetically.
In a hydrogen atom, if the electron is replaced by a particle which is 200 times heavier but has the same charge, how would its radius change?
Answer: 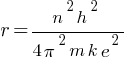
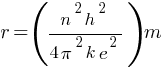
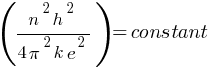
So, 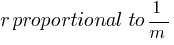
According to the above formula, the radius of a hydrogen atom is inversely proportional to the mass of an electron. so its radius will decrease to one upon 200 times the original radius with the increase in mass.
Why is the Distance of Closest Approach important in atomic interactions?
Answer : The strength and nature of the interaction between atoms are determined to some extent by the Distance of Closest Approach, which is why it is so important. It provides information about the potential energy between the atoms and can influence the likelihood of chemical reactions or other atomic processes occurring.
Are there any experimental techniques to measure the Distance of Closest Approach in atoms?
Answer: Yes, there are experimental techniques to measure the Distance of Closest Approach. Scattering experiments, such as those involving particle beams or X-ray scattering, can provide valuable information about the interaction between atoms and help determine the closest distance they approach each other.
Dual nature of radiation and matter Notes↗
Click above link for notes, MCQs and important questions.
Semiconductor Notes ↗
Click above link for notes, MCQs and important questions.
👉🖱 Bal Kavitaye बाल कविताएं best 5 poems