Composition and Size of Nucleus
The nucleus, a compact and dense area that contains the majority of an atom’s mass, is located in the centre of each atom. An atom’s properties and behaviour are greatly influenced by its composition and size of its nucleus. We will look into the fascinating world of the nucleus in this article, learning about its composition, structure, and significance to atomic science.
Composition of Nucleus
The nucleus of an atom consists of two sub-atomic particles, mainly protons and neutrons. The number of protons determines the atomic number of an atom, while the sum of protons and neutrons gives the atomic mass number.
Proton:
- A proton is a positive-charged particle that carries one unit of fundamental charge and is stable.
- The mass of a proton is 1.00727 u = 1.67262 × 10–27kg
Neutron:
- A neutron is a charge-less neutral particle.
- A free neutron, unlike a free proton, is unstable. It decays into a proton, an electron, and an antineutrino, and has a mean life of about 1000 s. It is, however, stable inside the nucleus.
- The mass of a neutron is nearly the same as the mass of a proton and is equal to 1.00866 u = 1.6749×10–27 kg
- James Chadwick discovered the neutron, for which he was awarded the 1935 Nobel Prize in Physics.
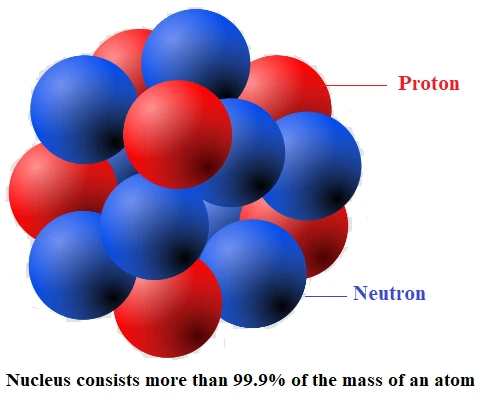
Table of Contents
Nucleus Size
The structure of the nucleus is governed by the strong nuclear force, a fundamental force of nature that binds protons and neutrons together. This force overcomes the electromagnetic repulsion between positively charged protons, keeping the nucleus stable and intact. The balance between the attractive strong nuclear force and the repulsive electromagnetic force ensures the structural integrity of the nucleus.
How Rutherford estimated size of nucleus?
Answer: By performing Rutherford’s scattering experiments on various elements, it is observed that the volume (V) of a nucleus is directly proportional to its mass number (A).
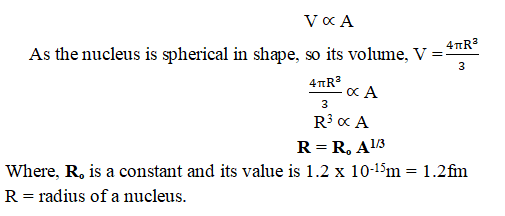
Depending on the atomic number, the nucleus’ size fluctuates. A nucleus’s diameter normally ranges between 1 and 10 femtometers (fm), where 1 fm is equal to 10-15 metres. Atoms with a higher atomic number have more protons and neutrons in their nuclei, which makes their nuclei larger.
Nuclear Force
Nuclear Force is the strongest interaction acting between the nucleons. It binds the protons and neutrons together inside the nucleus.
Cause of Nuclear force:
According to Yukawa (a Japanese Physicist), the nuclear force acts between the nucleons due to the continuous exchange of π-mesons between them.
Nuclear force properties:
- Short-range force has an effect up to a few fm.
- Nuclear forces may be attractive or repulsive in nature.
- The strength and nature of nuclear forces (either attractive or repulsive) depend on the separation between nucleons.
- It demonstrates the charge’s independence, i.e., the nuclear force between proton and proton, proton-neutron, and neutron-neutron is the same.
- It demonstrates the saturation effect, which states that nuclear force only acts between neighboring nucleons.
- It is a non-central force.
- It is a non-conservative force.
- It doesn’t follow the inverse square law.
- It is the strongest interaction among basic natural forces, and their relative strengths are, Fg : Fe : Fn = 1:1036:1038
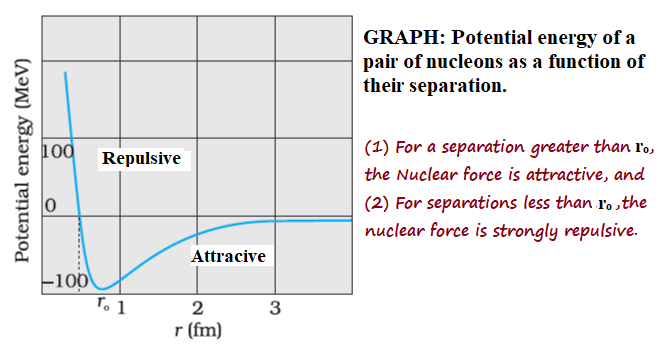
Variations of the Nuclear force with distance between Nucleons:
Variations of nuclear force with distance between nucleons can be understood on the basis of a graph between potential energy and separation between nucleons.
Einstein’s Mass-Energy Equivalence
According to Einstein’s Special Theory of Relativity mass and energy are interconvertible. Einstein suggested that mass is another form of energy and one can convert mass-energy.
E = mc2
The above relation is known as Einstein’s mass-energy equivalence. This equation relates the energy equivalent of mass m and c is speed of light in a vacuum, which is 3×108 m s–1.
Experimental verification of Einstein’s mass-energy relation has been achieved in the study of nuclear reactions amongst nucleons, nuclei, electrons, and other more recently discovered particles.
Question and Answers
How does the nuclear force work?
Answer: The nuclear force works by exchanging particles called mesons. These mesons mediate the interaction between nucleons (protons and neutrons) and provide the attractive force that overcomes the electromagnetic repulsion between positively charged protons.
Why do elements have different isotopes?
Answer: Elements have different isotopes because the number of neutrons in the nucleus can vary while the number of protons remains the same for a particular element. This variation in neutron number gives rise to isotopes with slightly different atomic masses.
How are isotopes used in medical applications?
Answer: Isotopes are used in medical applications such as diagnostic imaging and cancer treatment. Radioactive isotopes, known as radiopharmaceuticals, are used in techniques like positron emission tomography (PET)
What is the range of the nuclear force?
Answer: The nuclear force has a very short range, typically extending up to about 0.8 to 2.0 femtometers (fm). Beyond this range, the force diminishes rapidly.
What is the significance of the nuclear force?
Answer: The nuclear force is necessary for the stability and maintaining structure of nuclei. In absence of strong nuclear force, protons within the nucleus would repel each other, causing the nucleus to disintegrate. Thus nuclear force allows the formation of stable nuclei, enabling the existence of matter as we know it.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus, Composition and Size of Nucleus