Bohrs Atomic Model
Hello, readers 😊! Here you learn about the Bohr’s Atomic Model, Bohr’s Theory of Hydrogen Atom. In 1913 Bohr’s suggested a new model of atom which overcomes drawback of Rutherford’s atomic model. The discovery of Bohr’s atomic model was a milestone in the field of atomic structure models, which is more advanced and provides a comprehensive guide to atomic structure theory.
Bohr’s Atomic Model
Postulates of Bohr’s Atomic Model:
(1) Nuclear concept – An atom comprises a massive central core called the nucleus, surrounded by electrons in orbit. The electrostatic attraction between the electrons and the nucleus provides the necessary centripetal force for their revolution.
(2) Quantum condition – Electrons can only occupy orbits in which their angular momentum is a integral multiple multiple of h/2π, where h represents Planck’s constant. Thus, each permitted orbit have angular momentum,
L = mvr = nh/2π n = 1, 2, 3, ……………..
(3) Stationary orbits – During its revolution in permissible orbits, an electron does not emit energy. These non-radiating orbits are known as stationary orbits.
(4) Frequency condition – An atom can emit or absorb radiation in the form of discrete energy photons only when an electron transitions from a higher orbit to a lower orbit or from a lower orbit to a higher orbit, respectively.
If E1 and E2 represent the energies associated with these permitted orbits, the frequency (ν) of the emitted or absorbed radiation is given by,
hν = E2 – E1
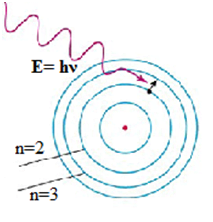
Table of Contents
Bohr’s Theory of Hydrogen Atom
According to Bohr’s theory of hydrogen atoms, a Hydrogen atom consists of a nucleus with a positive charge and a single negative charge electron, which revolves around it in a circular orbit.
- Quantization of Orbital Radius
- Quantization of Velocity
- Quantization of Energy
Quantization of Orbital radius
Consider an electron of mass m and charge e revolving in a circular orbit with velocity v around a positively charged (Ze) nucleus. A centripetal force (Fc) must be acting on an electron during its revolution, which is provided by an electrostatic force (Fe) between the nucleus and the electron.
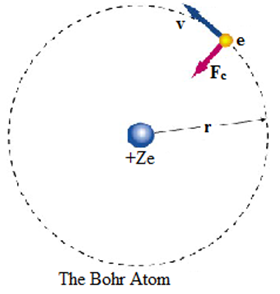
Centripetal force (Fc) = Electrostatic force (Fe)
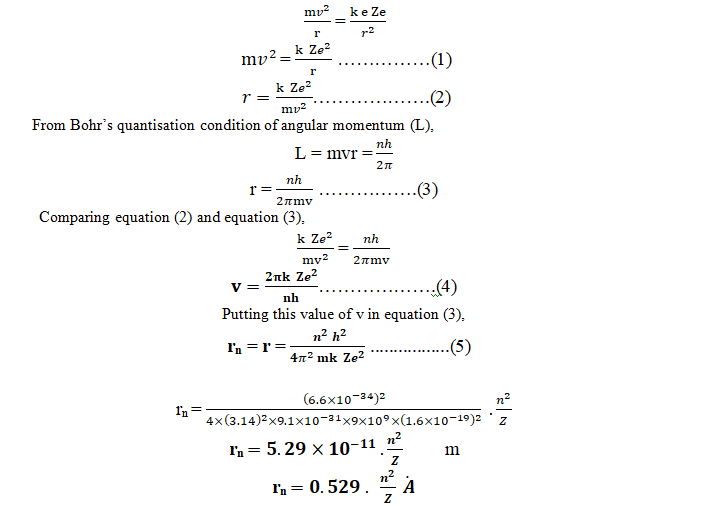
The radius of the orbit of an electron revolving around nucleus of H-atom in its ground state (n =1) is called Bohr’s radius. Bohr’s radius (ro) is a constant and its value is 5.29×10-11 m.
Quantization of Velocity
Quantization of velocity means electrons revolving around a nucleus can not move with any velocity. Their velocity has a discrete value and is a multiple of a certain fixed value.
Consider an electron of mass m and charge e revolving in a circular orbit with velocity v around a positively charged (Ze) nucleus. A centripetal force (Fc) must be acting on an electron during its revolution, which is provided by an electrostatic force (Fe) between the nucleus and the electron.
Centripetal force (Fc) = Electrostatic force (Fe)
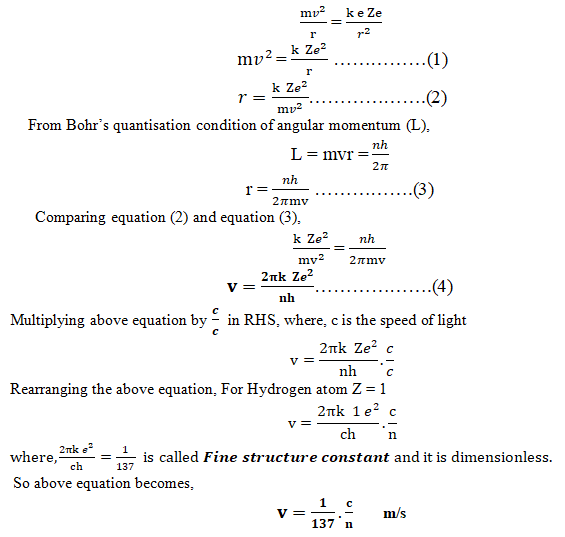
That is the velocity of an electron revolving around a nucleus in the nth orbit is quantized and is equal to c/n times of the Fine structure constant.
Quantization of Energy
The total energy of the electron revolving around nucleus is equal to sum of kinetic energy and potential energy.
Expression for the Kinetic energy of the moving object is K.E. = 1/2 mv2
For the revolution of an electron around the nucleus, a centripetal force (Fc) must be acted on it, which is provided by the electrostatic force (Fe) between the nucleus and the electron. So,
Centripetal force (Fc) = Electrostatic force (Fe)
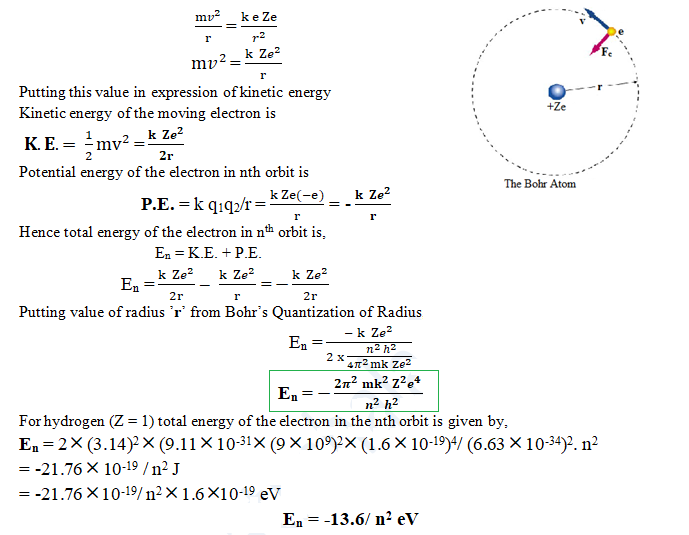
Thus, an electron can have only certain definite values (discrete value) of energy while revolving in different orbits. This is called energy quantization.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model, Bohrs Atomic Model,