Atoms and Nuclei
Atoms and nuclei are the fundamental building blocks of matter, forming the basis of everything we see around us. These tiny particles play a vital role in understanding the nature of the physical world. In this article, we will delve into the fascinating world of atoms and nuclei, exploring their structure, properties, and significance.
Over time, our understanding of the structure of an atom has changed. We have covered various atomic models in this session. You are going to learn the atomic structure in this chapter. J. J. Thomson’s atomic model comes first, followed by Rutherford’s atomic model, which is based on his well-known scattering experiment. We will also talk about Niels Bohr’s most sophisticated atomic model, which describes the electrical structure of an atom.
Atoms are the basic building blocks of matter. They are made up of three types of particles: protons, neutrons, and electrons.
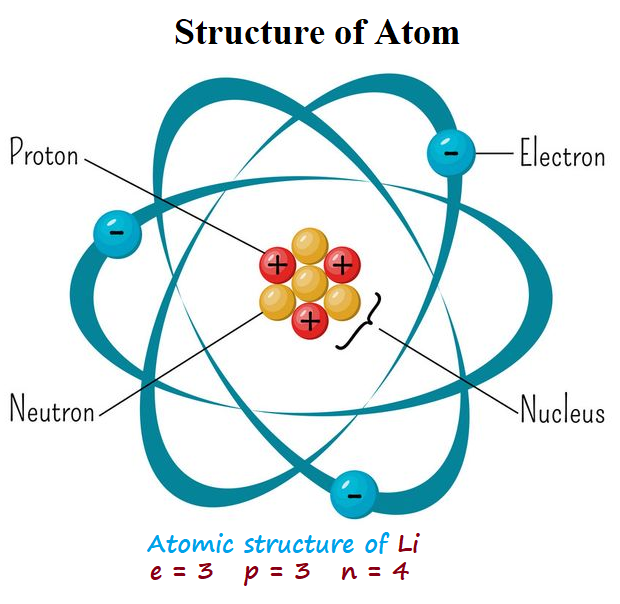
Protons are positively charged particles that are located in the nucleus, or the center, of an atom. The number of protons in an atom’s nucleus is called its atomic number and it uniquely identifies each element.
Electrons are negatively charged particles that orbit the nucleus. The number of electrons in an atom is equal to the number of protons, making it overall neutral in charge. Electrons occupy shells or energy levels around the nucleus.
Neutron is a subatomic particle found in the nucleus of an atom. It has no electric charge. The discovery of the neutron in 1932 was a significant milestone in atomic physics. Neutrons play important roles in nuclear reactions, scientific experiments, and medical imaging.
The structure of an atom is important in determining its chemical properties, such as reactivity, reactivity and ability to bond with other atoms. Atoms can bond with other atoms to form molecules and compounds, which make up all of the substances in the physical world.
Table of Contents
Atoms and Nuclei – INDEX
Thomson’s Atomic Model: An early model of an atom
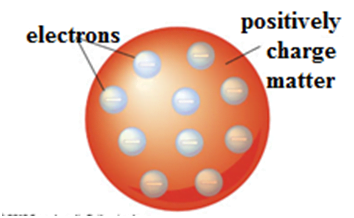
Thomson’s Atomic Model. It was the first atomic model, given by Sir Joseph John Thomson in 1898 also known as Plum Pudding Model or Watermelon Model.
According to Thompson, an atom is like a watermelon. The reddish part of watermelon is like the positively charged matter inside an atom, and the uniformly embedded seeds in watermelon are like electrons. An atom is a sphere of radius 10-10 m and is electrically neutral. He also discovered the electron, a negatively charged particle of an atom.
Limitation of Thomson’s Atomic Model:
- It could not explain the origin of spectral series of H atom.
- The Rutherford-Particle Scattering Experiment results contradict Thomson’s Atomic Model, which states that atoms are full, not empty, like watermelon.
Rutherford’s alpha-Particle Scattering Experiment
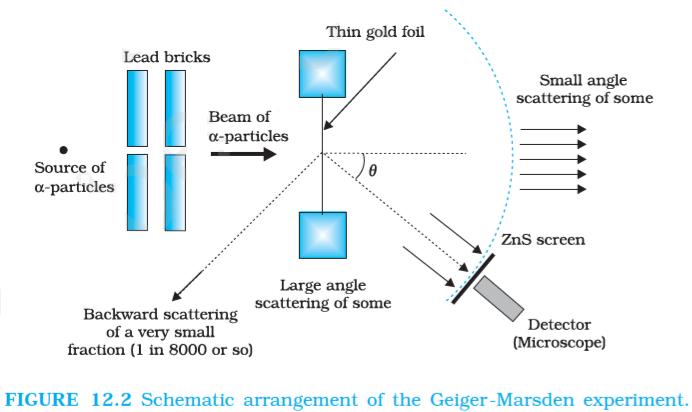
Rutherford’s alpha-Particle Scattering Experiment was carried out by H. Geiger and E. Marsden in 1911 on the suggestion of Ernst Rutherford. They directed a beam of 5.5MeV α-particles (α-particle is a nucleus of a helium atom carrying a charge of ‘+2e’ and mass equal to 4 times that of a hydrogen atom) emitted from a 214Bi83 radioactive source at a thin metal foil (10-7m) made of gold. The scattered α-particles were observed through a rotatable detector consisting of a ZnS screen and a microscope.
✒️ α-particles: α-particle is a nucleus of helium atom carrying a charge of ‘+2e’ and mass equal to 4 times that of hydrogen atom.
Trajectory of α-particles in the Coulomb field of a target nucleus:
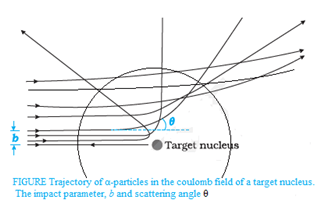
Graph between the number of scattered alpha-particles and the scattering angle:
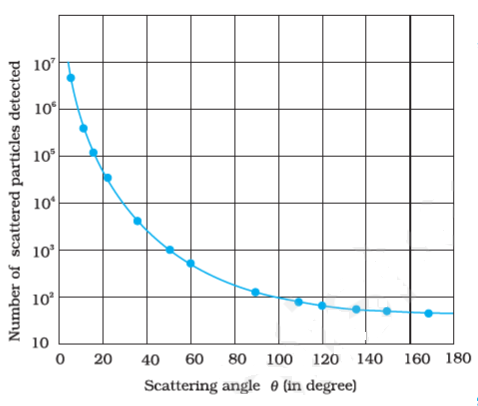
Rutherford’s alpha-Particle Scattering Experiment Observation and Conclusion:
| Sr. No. | Observation | Conclusion |
|---|---|---|
| 1 | Most of α- particle pass straight though gold foil or suffer only small deflection. | Most of the space within atom must be empty. |
| 2 | A very few α-particle about 1 in 8000, get deflected through a large angle, even more than 90⁰ . | All the positive charge and mass of the atom is concentrated in a very small region called nucleus. |
| 3 | An α-particle gets rebounded from the gold foil. | Nucleus of an atom can not be penetrated. |
Next Pages
👉🖱 What is Electric Flux ? Class 12 Important Topic
👉🖱 Electric field due to an Electric Dipole at Axial Point 12 Important topic
What is an atom?
Answer: An atom is the smallest unit of matter that retains the properties of an element.
Who is credited with the discovery of the electron?
Answer: J.J. Thomson is credited with the discovery of the electron.
What is the atomic number of an element?
Answer: The atomic number of an element is the number of protons in the nucleus of its atom.
What is an isotope?
Answer: An isotope is a variant of an element that has the same number of protons but a different number of neutrons.
What is the relationship between wavelength and frequency of an electromagnetic wave?
Answer: The wavelength and frequency of an electromagnetic wave are inversely proportional to each other.
Who proposed the planetary model of the atom?
Answer: Niels Bohr proposed the planetary model of the atom.
What is the charge of a proton?
Answer: The charge of a proton is positive (+1).
What is the charge of an electron?
Answer: The charge of an electron is – 1.6 × 10 -19 C .
What is an orbital?
Answer: An orbital is a region in an atom where an electron is most likely to be found.
What is the maximum number of electrons that can occupy an ‘s’ orbital?
Answer: The maximum number of electrons that can occupy an s orbital is two.
🖱️👉 Dual nature of radiation and matter
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei, Atoms and Nuclei
Nice site! if you need a book cover? would love to work with you! jbookcoverdesign.com follow me on instagram: @iamjoshuabruce @jbookcoverdesign
Thank you 😊!
Throughout the awesome pattern of things you actually secure an A just for effort. Where exactly you lost everybody ended up being in all the facts. As people say, the devil is in the details… And that could not be more accurate right here. Having said that, permit me inform you what did work. The writing is actually quite powerful which is possibly the reason why I am taking an effort to opine. I do not make it a regular habit of doing that. Next, although I can notice a jumps in reasoning you come up with, I am not really certain of how you seem to connect the details that help to make your conclusion. For now I will subscribe to your issue however trust in the foreseeable future you link your facts much better.
I appreciate the effort and time you’ve spent in putting together this information. Thank you for sharing this with us.
Can you write more about it? Your articles are always helpful to me. Thank you!
Thank you for your valuable feedback 😊. Sure I will.
Great beat ! I would like to apprentice while you amend your web site, how could i subscribe for a blog site? The account helped me a acceptable deal. I had been a little bit acquainted of this your broadcast provided bright clear concept
Thank you 😊
Thank you very much for sharing, I learned a lot from your article. Very cool. Thanks. nimabi
Sustain the excellent work and producing in the group!
I’m so in love with this. You did a great job!!
Good day! This post couldn’t be written any better! Reading through this post reminds me of my previous room mate! He always kept chatting about this. I will forward this article to him. Fairly certain he will have a good read. Many thanks for sharing!
I have read a few good stuff here. Definitely price bookmarking for revisiting. I wonder how much attempt you place to make this type of fantastic informative website.
Your point of view caught my eye and was very interesting. Thanks. I have a question for you.
Your article helped me a lot, is there any more related content? Thanks!
Thank you for your sharing. I am worried that I lack creative ideas. It is your article that makes me full of hope. Thank you. But, I have a question, can you help me?
Thanks for sharing. I read many of your blog posts, cool, your blog is very good.