Electric Charge
Charge is the property of a material due to which an electrostatic force acts between two charge bodies.
Unit of charge:
The SI unit of charge is C (coulomb).
Definition of 1 coulomb: One Coulomb of charge is defined as the amount of electric charge transported by a constant current of one ampere (A) in one second.
Other units of charge:
Electrostatic Unit of Charge (esu) / Statcoulomb / Franklin (Fr) – Oldest unit of charge, used to measure magnitude of charge.
1 esu = 3.33564 × 10-10 C.
Table of Contents
REVISION FACTS
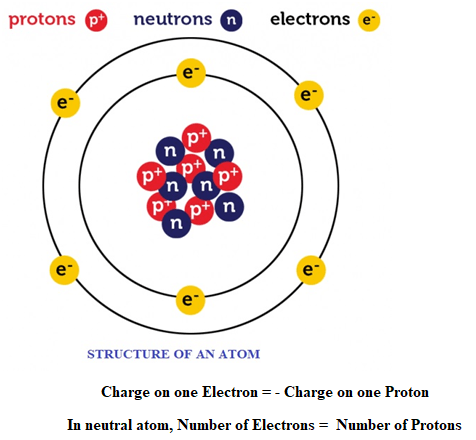
About atom: All matters surrounding us are made from atoms. Before we study more about charge we should know some basic concept of atom. An atom consists of mainly 3 subatomic particles namely Proton, Electron and Neutron.
- Protons: It is positively charge particle, which lies in nucleus of an atom. Charge on one proton is +1.6 × 10-19 C.
- Electron: It is negatively charge particle, which revolve surrounding nucleus of an atom. Charge on one electron is -1.6 × 10-19 C.
- Neutron: It is a neutral (charge less) particle which lies inside nucleus.
About charge:
- Charge on electrons and protons have equal magnitudes but opposite sign.
- Charge on one Electron = -1.6 × 10-19 C
- Charge on one Proton = +1.6 × 10-19 C
- Charge on one Electron = – Charge on one Proton
- An atom is always neutral as it contains equal number of electrons and protons. When it becomes charged (ions) number of electron is not equal to number of protons. For example:
K (neutral atom) ——–→ K+ (positive Ion) + 1e– (released electron)
- Charge can be positive or negative. Like charges repel, opposite charges attract: Two particles with the same charge will repel each other, while two particles with opposite charges will attract each other.
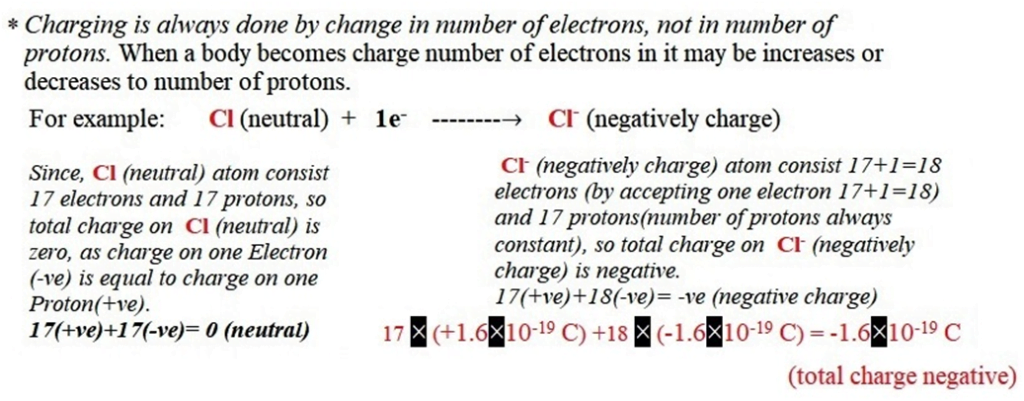
- Charging is always done by changing the number of electrons, not the number of protons. In the electrically neutral state of the body, the number of electrons is equal to the number of protons. When a body becomes charged, the number of electrons changes. It may be increases or decreases.
Types of charge:
Charge is of two types (1) Positive charge (2) Negative charge
(1) Positive charge: If a body gives/release electrons, it becomes positively charge.
For ex. Na (neutral) ——–→ Na+ (positively charge) + 1e–(released electron)
(2) Negative charge: If a body accepts electrons, it becomes negatively charge.
For ex. Cl (neutral) +1e–——–→ Cl– (negatively charge)
Key Concept ☞ : The assignment of positive charge is purely a convention. It doesn’t mean that negative charge is less than positive charge i.e. +2C and -2C are same amount of charge. +2C of charge means body acquires this charge by donating/releasing electrons, while,-2C of charge means body acquires this charge by accepting electrons.
3 Fundamental Properties of Electric Charge
Electric charge is a fundamental property of matter that describes the presence of an electrical property on an object or particle. All known matter is composed of atoms, which in turn are composed of positively charged protons, negatively charged electrons, and uncharged neutrons. The electric charge of an object or particle is determined by the number of protons and electrons it possesses.
Electric charge is a fundamental property of matter and plays a crucial role in many physical processes. Here are 3 Fundamental properties of Electric charge:
(1) Quantization of charge:
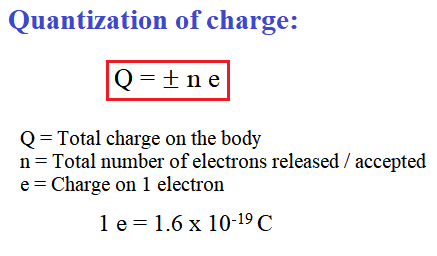
Charge is always quantized. Total charge on a body is equal to integral multiples of charge on a single electron.
(2) Conservation of charge:
In an isolated system, the total amount of charge remains constant. A net charge can neither be produced nor destroyed, it can transfer from one body to another.
When a rubber balloon is rubbed against a person’s hair, electrons are transferred from the hair to the balloon, giving the balloon a net negative charge and the hair a net positive charge. Again, the total charge of the system remains constant, because the amount of charge transferred from the hair to the balloon is equal in magnitude but opposite in sign to the charge transferred from the balloon to the hair.
(3) Additive nature of charge:
The total charge on a system is equal to the algebraic sum of all the individual point charges that exist within that system.
For example, if two objects with charges of +3 unit and -2 unit are brought together, the total charge of the system will be +1 unit, which is the sum of the individual charges.
Overall, these 3 fundamental properties of electric charge – additive nature, quantization, and conservation – are fundamental to our understanding of the behavior of charged particles and the interactions between them. They provide a framework for describing and predicting the behavior of electric and magnetic fields, as well as the behavior of particles at the atomic and subatomic level.
Detection and measurement of charge: Gold-leaf electroscope
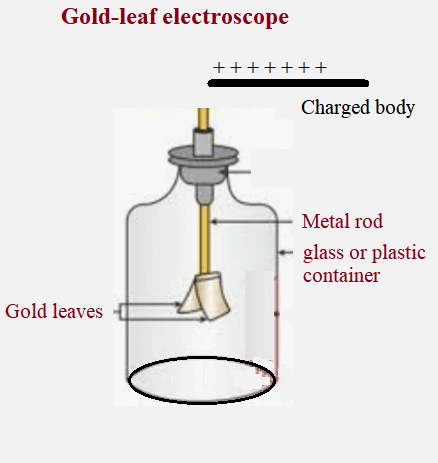
Gold-leaf electroscope:
A gold-leaf electroscope is a scientific instrument used to detect and measure electric charges. It consists of a metal rod or wire with thin strips of gold foil attached to the bottom, enclosed in a glass or plastic container. When a charged object is brought near the electroscope, the gold leaves become charged and repel each other, causing them to diverge. The degree of leaf divergence provides an indication of the strength and polarity of the charge. The gold-leaf electroscope is sensitive and can detect even small amounts of charge, but is subject to interference from environmental factors.
👉👁️🗨️ Video lecture: Electric Charge, Charge and its properties:
Questions related to the Topic
What is the property of charge that determines how strongly it interacts with electric fields?
Ans: The property of charge that determines how strongly it interacts with electric fields is known as electric charge magnitude.
What is the property of charge that determines whether it is attracted to or repelled by other charged objects?
Ans: The property of charge that determines whether it is attracted to or repelled by other charged objects is known as electric charge polarity.
What is the property of charge that determines how easily it can move through a material?
Ans: The property of charge that determines how easily it can move through a material is known as electric charge mobility.
What is the property of charge that determines how much energy is required to move it through a potential difference?
Ans: The property of charge that determines how much energy is required to move it through a potential difference is known as electric charge energy.
What is the property of charge that determines how long it can be stored in a material or object?
Ans: The property of charge that determines how long it can be stored in a material or object is known as electric charge decay time.
What is the property of charge that determines whether it is constant in a closed system?
Ans: The property of charge that determines whether it is conserved in a closed system is known as electric charge conservation.
What is the property of charge that determines how it is distributed within a material or object?
Ans: The property of charge that determines how it is distributed within a material or object is known as electric charge density.
Define charge and provide examples of objects that can have a net electric charge.
Charge is a fundamental property of matter that causes it to experience a force in the presence of an electric field. Objects that can have a net electric charge include electrons, protons, ions, and some macroscopic objects such as balloons that have been rubbed with a wool cloth.
Describe the difference between conductors and insulators in terms of their ability to hold and transfer electric charge.
Conductors are materials that allow electric charge to flow freely, while insulators are materials that do not allow charge to flow easily. Conductors typically have free electrons that can move around and carry charge, while insulators have tightly bound electrons that are less able to move.
What is Coulomb’s law, and how is it used to calculate the electric force between charged particles?
Coulomb’s law is a fundamental law of physics that describes the electric force between two charged particles. The law states that the force between two point charges is proportional to the product of their charges and inversely proportional to the square of the distance between them.
Explain the concept of electric potential, and how it relates to charge.
Electric potential is the amount of work required to move a unit of charge from one point to another in an electric field. It is related to electric charge through the equation V = W/q, where V is electric potential, W is work, and q is electric charge.
How can the transfer of electric charge be induced through the process of electrostatic induction?
Electrostatic induction is a process in which a charged object is brought close to an uncharged object, causing the charges in the uncharged object to redistribute. This can result in a net transfer of charge from one object to another.
Discuss the relationship between charge and electric current, and provide examples of devices that utilize this relationship.
Electric current is the flow of electric charge through a conductor, and is related to charge through the equation I = q/t, where I is current, q is charge, and t is time. Examples of devices that utilize this relationship include batteries, generators, and electric motors.
What is an electric field, and how does it relate to the concept of electric charge?
An electric field is a region of space in which a charged particle experiences a force. It is related to charge through the equation E = F/q, where E is electric field, F is electric force, and q is electric charge.
How do charges interact with magnetic fields, and what is the relationship between electric and magnetic forces?
Electric charges interact with magnetic fields through the Lorentz force, which describes the force experienced by a charged particle in a magnetic field. The relationship between electric and magnetic forces is described by the electromagnetic force.
MY YouTube Channel Link : 👉🖱 https://www.youtube.com/channel/UCGpC7nWE0-bBv9I53MM8qjQ
Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge
Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge, Fundamental Properties of Electric Charge